Unexplained Hypoglycemia: A Focused Approach to Finding the Cause
Most episodes of recurrent hypoglycemia occur in patients with diabetes mellitus and can be prevented by changes in medication, diet, or activity. However, persistent, unexplained hypoglycemia can indicate a potentially grave, often treatable, underlying disorder, such as adrenal or pituitary insufficiency. Medication errors are another common cause of unexplained hypoglycemia. A focused laboratory workup is essential; obtain a plasma or serum glucose level and serum insulin and C-peptide levels. Insulin levels should be suppressed (less than 6 μIU/mL) when the glucose level is below 60 mg/dL. “Normal” or high insulin and C-peptide levels suggest excessive endogenous insulin production. Another useful value is the serum β-hydroxybutyrate level. Patients with insulin-mediated hypoglycemia typically have β-hydroxybutyrate concentrations below 2.7 mg/dL.
Hypoglycemia is a common event that typically occurs in persons with diabetes mellitus. In this context, hypoglycemia usually results from an imbalance among diabetic therapy, level of activity, and dietary intake. Therefore, management of hypoglycemia has become “scripted”: glucose administration, followed by adjustments in insulin or oral medications or diet. The simplicity of this approach can be deceptive, however. Practitioners may underestimate the impact of other hypoglycemic disorders that are not the direct result of diabetes or its treatment regimen. In this setting, hypoglycemia can indicate a serious disturbance in glucose regulation and can recur with devastating consequences. Determining the cause of hypoglycemic “outliers,” therefore, is critical. The potentially life-threatening consequences of sudden, unexpected hypoglycemia may endanger not only the affected person but others as well (eg, hypoglycemia in a driver of a motor vehicle). Here we present 3 examples of hypoglycemia that occurred in persons without diabetes (Cases 1, 2, and 3). The low blood glucose levels were recognized, but they were not evaluated fully at initial presentation. The episodes were then followed by potentially dangerous events. In our discussion of these cases, we will describe the appropriate workup and management of recurrent hypoglycemia that is unrelated to diabetes or diabetic treatment.
_______________________________________________________________________________________________________________________________________________
Related Content
Iatrogenic Hypoglycemia: Identification, Troubleshooting, and Precautions
Sulfonylurea-Induced Hypoglycemia: The Case Against Glyburide
_______________________________________________________________________________________________________________________________________________
A WORKING DEFINITION OF HYPOGLYCEMIA
Hypoglycemia may be defined as a clinical syndrome in which low serum glucose levels lead to symptoms and signs of neuroglycopenia (eg, an altered sensorium and seizures) and sympathoadrenal activation.1 Glucose concentrations of 60 mg/dL or less may cause autonomic symptoms and those below 50 mg/dL may cause impaired brain function.1
Case 1 – Nondiabetic Woman With Recurrent Episodes of Hypoglycemia
Clinical background. A 36-year-old woman had 2 episodes of hypoglycemia. During the first episode, the glucose level was 12 mg/dL (capillary) and 26 mg/dL (serum); during the second episode, the capillary glucose level was 37 mg/dL, but the serum level was not correspondingly low. In both instances, she was treated with intravenous dextrose, and the hypoglycemic symptoms and signs resolved. Unfortunately, the resolution was only temporary. Although the patient was not known to have diabetes, further workup of the hypoglycemia was not pursued.
After the second episode, the patient was hospitalized. Several days later, she experienced cardiovascular collapse. Hypopituitarism with severe, secondary adrenal insufficiency was diagnosed based on a morning plasma adrenocorticotropic hormone (ACTH) level of 5 pg/mL and a serum cortisol level of less than 1 μg/dL.
Case discussion. On admission, the patient’s thyroid-stimulating hormone (TSH) level was 6.11 μIU/mL (normal range, 0.5 to 5.0 μIU/mL) and the free thyroxine (T4) level was 0.5 ng/dL (normal range, 0.9 to 2.4 ng/dL). These values were interpreted as discordant for a diagnosis of either primary hypothyroidism or the sick euthyroid syndrome (decreased thyroid function as a result of a non-thyroidal illness), but possibly consistent with secondary hypothyroidism. With a low free T4 level of 0.5 ng/dL, the corresponding TSH level should be higher than 6.11 μIU/mL in a patient with primary hypothyroidism. Conversely, during secondary hypothyroidism, the TSH and free T4 levels should both be low. In the setting of secondary adrenal insufficiency, the likelihood of concomitant secondary hypothyroidism is increased.
A fundamental rule in the interpretation of endocrine laboratory results is the concept of “inappropriately normal” values. Hormone levels are always regulated in concert with feedback. Thus, they should never be interpreted in isolation. That is, they must be interpreted relative to the counter-regulating factors (eg, low thyroid function increases the TSH level in an attempt to stimulate the failing pituitary gland).
Outcome of this case. Because of this patient’s poor prognosis, she did not undergo imaging studies to further delineate her pituitary status. She had severe anoxic encephalopathy, necessitating ventilator dependence and enteral nutrition for life, and thus was not considered to be a candidate for any radical intervention even if a pituitary cause was uncovered. She was given medical treatment, which included oral glucocorticoids. Take-home message. This case illustrates a life-threatening situation that was precipitated by secondary (pituitary ACTH-deficient) adrenal insufficiency. The previous episodes of “unexplained hypoglycemia” were warnings that, at least initially, were not evaluated further. Unfortunately, the patient sustained severe, residual anoxic encephalopathy as a result of recurrent and prolonged hypoglycemia. Greater vigilance during her previous hypoglycemic episodes may have prevented this outcome. This case represents the most extreme example of the serious consequences of unrecognized, recurrent hypoglycemia.
As a general rule, confirmed glucose values below 50 mg/dL deserve further evaluation,2 but suggestive symptoms without low serum glucose levels are not diagnostic. The Whipple triad summarizes the minimum criteria required for a true hypoglycemic disorder:
- Symptoms and signs consistent with hypoglycemia.
- A documented low serum glucose level at the time of the symptoms and signs.
- Resolution of symptoms and signs with correction of the hypoglycemia.
CLASSIFICATION OF HYPOGLYCEMIA
Hypoglycemia may be a manifestation of diverse pathologies. These have been variably classified into insulin-dependent or insulin-independent, and preprandial or postprandial (also called reactive), hypoglycemic disorders. Either classification has limitations. The former describes pathogenesis but does not provide information about causation; some researchers consider the latter classification to be ambiguous because certain causes of hypoglycemia (eg, insulinoma) can cause both preprandial and postprandial symptoms. Thus, a classification based on the appearance of a nondiabetic patient, whether healthy or ill, has been proposed.3 The healthy-appearing patient has either no known diseases or well-compensated problems; “sickappearing” patients include those hospitalized for another illness or those with coexistent, decompensated diseases (eg, progressive renal failure, sepsis, congestive heart failure, or liver failure). Although such an approach may offer some clues to the underlying cause of hypoglycemia, remember that some causes can be associated with either a sick- or a healthy-appearing person. For example, persons with adrenal or pituitary insufficiency, or those whose hypoglycemia stems from a drug error, can appear healthy or ill depending on the clinical circumstances. Also, adrenal insufficiency can develop in persons with diabetes mellitus. Thus, appropriate evaluation is necessary regardless of the classification of hypoglycemia in order to confirm the pathology and to formulate a treatment plan.
Continue to next page for Case 2
Case 2 – Seizures in an Older Man With Rheumatoid Arthritis
Clinical background. Prednisone (Deltasone) was prescribed for joint inflammation in a 67-year-old man with rheumatoid arthritis. However, because of a pharmacy error, glyburide (DiaBeta) was dispensed inadvertently. Subsequently, he experienced 3 tonic-clonic convulsions, one of which was accompanied by a blood glucose level of 28 mg/dL. The discovery of the mislabeled medication bottle and the correction of the error eliminated the hypoglycemia and seizures. The patient had no permanent sequelae.
Case discussion. The key test in this patient demonstrated elevated levels of endogenous serum insulin during an episode of hypoglycemia. The serum glucose level was 40 mg/dL; serum insulin, 10 μIU/mL (fasting range, 5 to 20 μIU/mL); and serum C peptide, 2.0 ng/mL (range, 1.1 to 5.0 ng/mL). In this instance, the insulin and C-peptide results were both inappropriately “normal” (hypoglycemia should suppress insulin endogenous secretion). Therefore, both values were relatively increased and confirmed the endogenous secretion of insulin. A test for serum sulfonylureas was negative. Sulfonylurea measurements can be misleading, especially when the assay is applied to second-generation sulfonylureas (such as glyburide). Newer assays are more reliable, but they were not available at the time of this patient’s episode of hypoglycemia. A CT scan of the pancreas was obtained in case an insulinoma (also associated with elevated insulin and C-peptide levels despite hypoglycemia) was present. No evidence of a pancreatic tumor was found.
Take-home messages. This case contains several important lessons:
- First, anyone can make an error in the complex environment of contemporary medical practice. Faulty systems lead to mistakes. The need for checks and counterchecks for things as simple as prescription medications can never be minimized.
- Second, review of a patient’s medication list, as well as the bottles and pills contained in them, is essential. Every effort should be made to obtain the list directly from the patient, and, if possible, the actual bottles and tablets should be examined and occasionally counted. In this case, the pharmacy records and the label on the bottle indicated prednisone, but the tablets inside were glyburide. Critical information was obtained by examining the patient’s medications directly.
- Third, mistakes are common. Hypoglycemia is more often related to medication errors than to a true endocrine phenomenon such as an insulinoma or Addison disease.
Patient safety has become a paramount issue for primary care (Box). In the 2009 Safety Standards published by the Joint Commission for the Accreditation of Hospitals, medication safety is prominent.8

CLINICAL MANIFESTATIONS
Serious hypoglycemia manifests with symptoms and signs related to sympathoadrenal activation and neuroglycopenia. Sympathoadrenal activation includes sweating, tachycardia, tachypnea, anxiety, tremulousness, and nausea. Neuroglycopenic symptoms and signs consist of altered vision, fatigue, dizziness, headache, confusion, mental status changes, seizures, coma, and potentially death. Not all symptoms or signs are present in each patient who has hypoglycemia. However, the pattern of an individual patient’s complications is usually consistent during repeated episodes.
DIAGNOSIS
When patients present with symptoms or signs of hypoglycemia or have a low blood glucose level, it is important to initiate a focused laboratory workup. This approach is imperative in those who are not known to have diabetes or in persons with diabetes who have not responded to appropriate attempts to remedy their hypoglycemia (adjustments in medications or diet, for example). Serum glucose values below 60 mg/dL, accompanied by a history of symptoms or signs consistent with hypoglycemia, suggest an underlying, nondiabetic hypoglycemic pathology. A glucose value below 50 mg/dL with hypoglycemic symptoms or signs mandates further investigation. Initiate diagnostic studies while the patient is hypoglycemic.4 Obtaining additional serum samples at the initial hypoglycemic presentation, especially in patients who are not known to have diabetes, can facilitate diagnostic testing. If the suspicion of a hypoglycemic disorder is sufficiently high and symptomatic episodes infrequent, a supervised 72-hour fast in consultation with an endocrinologist to confirm hypoglycemia and determine a specific cause may be warranted (Table). In the workup of hypoglycemia, the following laboratory studies should be included:
- A plasma or serum glucose level: Capillary glucose levels below 60 mg/dL are suggestive but not always reliable. A low plasma or serum glucose level should be confirmed on at least 2 more occasions.
- Serum insulin and C-peptide levels: Insulin levels should be suppressed (less than 6 μIU/mL) when the glu-cose level is below 60 mg/dL.

The presence or absence of insulin during episodes of hypoglycemia helps identify the specific cause. Insulin-induced hypoglycemia can then be further divided into those with inappropriate endogenous or exogenous sources of insulin based on the C-peptide levels, because C peptide is found only with endogenous insulin secretion. Another informative value is the serum β-hydroxybutyrate level. β-Hydroxybutyrate is a ketone, and insulin inhibits ketone production. Therefore, hypoglycemia mediated by high insulin- like activity (in rare cases, other substances and medications may have actions similar to those of insulin) should be associated with low β-hydroxybutyrate levels. Patients with insulin-mediated hypoglycemia typically have β-hydroxybutyrate concentrations below 2.7 mg/dL.2 Further workup is contingent on the results of these initial, focused laboratory studies. At this point, it is important to consult an endocrinologist because the causes discussed here are not common and may require specialized diagnostic and therapeutic interventions.
Continue to next page for Case 3
Case 3 – Young Woman With Recurrent Seizures
Clinical background. A 24-year-old woman with newonset generalized seizures was hospitalized. An electroencephalogram and an MRI scan showed no neurological cause of the seizures. Hypoglycemia (serum glucose level, 30 mg/dL) was documented and treated but was not evaluated further. She later presented with another seizure and a blood glucose level of 20 mg/dL. Measurement of the adrenocorticotropic hormone (ACTH) level (1062 pg/mL) and the serum cortisol level (0.5 μg/dL) led to a diagnosis of primary adrenal insufficiency (Addison disease). The seizures ended after she received corticosteroid therapy.
Case discussion. Hypoglycemia was considered the probable cause of this patient’s seizure activity. The seizures and episodes of hypoglycemia occurred each time she experienced a flu-like illness. There appeared to be no relationship between seizure activity and meals, activity, or stress.
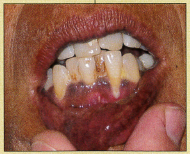 After further questioning, the patient reported that she had lost 87 to 95 lb over the past 2 to 3 years. A focused physical examination revealed the hyperpigmentation of Addison disease. (The Figure shows hyperpigmented gingiva in another patient with primary adrenal insufficiency). This patient’s insulin and C-peptide levels were undetectable, a finding consistent with the diagnosis of Addison disease. Because endogenous insulin secretion was not responsible for the hypoglycemia, it was suppressed. The culprit was a lack of adrenal production of steroids.
After further questioning, the patient reported that she had lost 87 to 95 lb over the past 2 to 3 years. A focused physical examination revealed the hyperpigmentation of Addison disease. (The Figure shows hyperpigmented gingiva in another patient with primary adrenal insufficiency). This patient’s insulin and C-peptide levels were undetectable, a finding consistent with the diagnosis of Addison disease. Because endogenous insulin secretion was not responsible for the hypoglycemia, it was suppressed. The culprit was a lack of adrenal production of steroids.
The ACTH level was markedly elevated because the pituitary was trying to stimulate a failing adrenal gland, but the cortisol level was extremely low. There was no response to cosyntropin (synthetic ACTH), 250 μg given intravenously. This workup confirmed the diagnosis of primary adrenal insufficiency.
The patient’s thyroid-stimulating hormone (TSH) level was 50 μIU/mL; free thyroxine (T4) level, 1.1 ng/dL; and triiodothyronine uptake, 26.6%. These values indicate primary hypothyroidism in addition to adrenal insufficiency (a low T4 level despite an elevated TSH level). Markedly increased levels of antimicrosomal antithyroid antibodies suggested that an autoimmune disorder was responsible for the hypothyroidism. The presence of autoimmune primary hypothyroidism makes it likely that the primary adrenal insufficiency also had an autoimmune cause.
Take-home message. This case highlights the importance of the “degree” of hypoglycemia in a patient with seizures. Blood glucose levels below 55 mg/dL suggest that hypoglycemia is responsible for the seizures.
CAUSES OF UNEXPLAINED HYPOGLYCEMIA
In the cases presented in this article, insulinoma as a potential cause of a hypoglycemic presentation is conspicuous by its absence. This omission was not intentional. Insulinoma is, and remains, an important cause of hypoglycemia, and it should be suspected when the initial workup suggests excessive endogenous insulin production (inappropriately “normal” or high insulin and C-peptide levels, with low β-hydroxybutyrate levels, all in the presence of low serum glucose). The diagnosis was entertained and ruled out in each case of hypoglycemia discussed in this article. However, contrary to popular belief, insulinoma is one of the less common causes of hypoglycemia. Other endocrine problems (eg, primary and secondary adrenal insufficiency, hypothyroidism), metabolic problems, and medication errors have become more common. We refer readers who are interested in learning more about insulinomas to review articles by Grant,5 Doherty,6 and Hirshberg and colleagues.7
1. Service FJ. Hypoglycemic disorders. N Engl J Med. 1995;332:1144-1152.
2. Service FJ. Diagnostic approach to adults with hypoglycemic disorders. Endocrinol Metab Clin North Am. 1999;28:519-532, vi.
3. Service FJ. Classification of hypoglycemic disorders. Endocrinol Metab Clin North Am. 1999;28: 501-517, vi.
4. Jordan RM. Endocrine emergencies. Med Clin North Am. 1983;67:1193-1213.
5. Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19:783-798.
6. Doherty GM. Multiple endocrine neoplasia type 1. J Surg Oncol. 2005;89:143-150.
7. Hirshberg B, Cochran C, Skarulis MC, et al. Malignant insulinoma: spectrum of unusual clinical features. Cancer. 2005;104:264-272.
8. Joint Commission for the Accreditation of Hospitals. 2009 National Patient Safety Goals. http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed July 7, 2008.
9. Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human: Building a Safer Health System. Institute of Medicine. Committee on Quality Health Care in America. Washington, DC: National Academies Press; 2000.
10. Leape LL, Brennan TA, Laird NM, et al. The nature of adverse events in hospitalized patients: results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377-384.
11. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients: results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324:370-376.
12. Cina JL, Gandhi TK, Churchill W, et al. How many hospital pharmacy medication dispensing errors go undetected? Jt Comm J Qual Patient Saf. 2006;32:73-80.
13. Poon EG, Cina JL, Churchill W, et al. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology
in the pharmacy. Ann Intern Med. 2006;145: 426-434.
14. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49: 196-205.
15. Gandhi TK, Kachalia A, Thomas EJ, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med. 2006;145:488-496.


