Peer Reviewed
Splenic Abscess Due to Salmonellosis
Authors:
Tara J. Scheufler, DO, and Angela M. Palitto, DO
Citation:
Scheufler TJ, Palitto AM. Splenic abscess due to salmonellosis. Consultant. 2017;57(9):530-533.
A 59-year-old man with alcoholic liver cirrhosis, chronic pancreatitis with pseudocysts, likely type 1 diabetes mellitus due to chronic pancreatitis, and current alcohol and tobacco use presented to our emergency department with a chief concern of severe generalized weakness along with anorexia, severe left upper-quadrant pain, a recent 9-kg weight loss, near-syncope, and subjective fevers, chills, and sweats. He also reported having had several days of bloody diarrhea a few weeks prior to presentation, for which he did not seek treatment.
He denied having eaten raw chicken or other raw meat, having eaten undercooked frozen foods, and owning a reptile or a bird. He reported having eaten at a fast-food restaurant a month ago; although the health department had reported that a local restaurant had been the source of a concurrent Salmonella outbreak, he denied having eaten at that restaurant. He denied that any of his family or friends had recently had episodes of diarrhea.
On examination, the patient was mildly cachectic and moderately ill-appearing, with dry mucous membranes. Vital signs and cardiovascular examination findings were normal. The lungs were clear to auscultation. He had left upper-quadrant tenderness to palpation without an acute abdomen and without ascites.
Diagnostic tests
Laboratory test results included the following values: white blood cell (WBC) count, 11,600/µL, with Döhle bodies and toxic granulation; creatinine, 9.6 mg/dL; blood urea nitrogen, 152 mg/dL; sodium, 130 mEq/L; anion gap, 29 mEq/L; bicarbonate, 9 mEq/L; lactate, 3.2 mg/dL; international normalized ratio, 1.4; alkaline phosphatase, 205 U/L; alanine aminotransferase, 118 U/L; and lipase, 457 U/L. The patient was admitted for acute renal failure from dehydration, sepsis from an unknown source, and poorly controlled left upper-quadrant pain.
Blood culture results were positive for gram-negative bacteria on hospital day 2. Abdominal ultrasonography scans showed a 3.0 × 2.5-cm mass in the spleen with surrounding free fluid. Computed tomography (CT) scans of the abdomen and pelvis without contrast revealed a new 3-cm lesion in the midspleen, a 10 × 4-cm fluid collection posterior to the spleen and anterior to the diaphragmatic peritoneal reflection, and the presence of mild jejunitis (Figure 1). Nodularity and reactive lymph nodes were also noted around the spleen, along with splenic varices.

Figure 1: Initial CT appearance of a 3-cm, midsplenic, intraparenchymal abscess, along with a 10 × 4-cm uid collection posterior to the spleen in the peritoneal recess.
Surgical and infectious disease specialty consultations were obtained for the splenic lesion. His Child-Pugh liver-disease classification score was 6, correlating with a life expectancy of 15 to 20 years and an abdominal surgical perioperative mortality risk of 10%. A transthoracic echocardiogram showed a normal ejection fraction and no vegetations.
A finding of Salmonellosis
Blood culture results revealed Salmonella group B, with further subtyping completed by the Ohio Department of Health showing Salmonella ser Typhimurium (type B in the previous classification system); this serovar was resistant only to ampicillin. Initially, the man received intravenous (IV) ceftriaxone, 2000 mg daily. Because of his cirrhotic liver disease, CT-guided percutaneous peritoneal-fluid drainage with drain placement was performed in lieu of splenectomy. Fluid culture results revealed the same Salmonella serovar identified in the blood cultures.
CT scans of the abdomen and pelvis with contrast on day 3 after drain placement showed a significant decrease in the size of the perisplenic fluid collection but no change in the size of the splenic parenchymal abscess (Figure 2). Results of repeated blood culture tests were negative for infection. The patient’s creatinine level returned to baseline, his sepsis resolved, and he was discharged home on a 4-week course of levofloxacin.
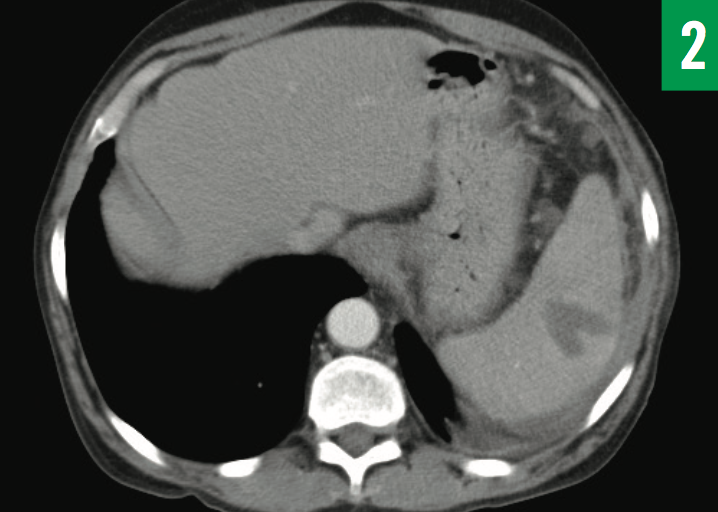
Figure 2: CT appearance of the splenic abscess 3 days after aspiration and drainage catheter placement. The size of the intraparen- chymal abscess was unchanged at 3.7 × 2.6 cm, but the fluid collection posterior to the spleen had signi cantly decreased.
The patient required readmission 5 days after discharge due to a recurrence of severe generalized weakness, fever, and sepsis. His WBC count was 13,900/µL, with 90.5% neutrophils and 0% bands, and he had a maximum temperature of 38°C during this second hospital stay. He was placed on IV levofloxacin and then IV ceftriaxone alone for 5 days prior to discharge. Surgical and infectious disease consultations were again obtained. CT scans with contrast revealed a decrease in size of the intraparenchymal splenic abscess, measuring 3.2 × 2.4 cm, as well as resolution of the pus collection posterior to the spleen (Figure 3).
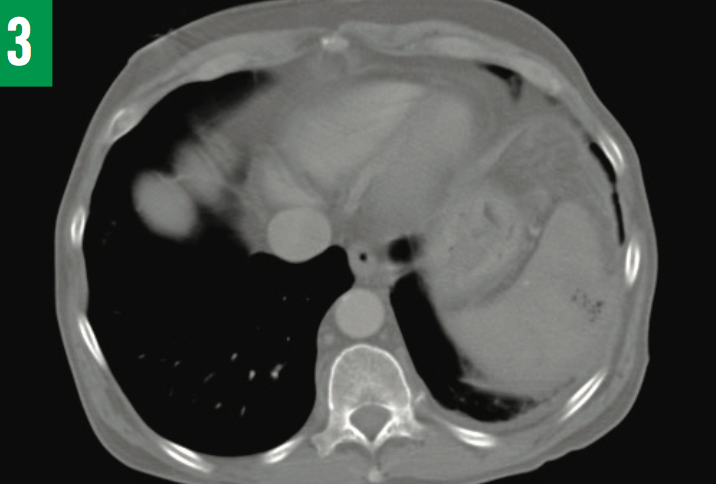
Figure 3: CT appearance of the splenic abscess at the time of the second hospital admission (and after 13 days of antibiotic treatment). The size had decreased to 3.2×2.4 cm. The cath- eter drain was still in place posterior to the spleen in peritoneal space (not visible on this CT image slice).
Once his sepsis had resolved, the patient was again discharged, with the original drain in place, and was continued on IV levofloxacin, 750 mg daily, and then IV ceftriaxone, 2000 mg daily, for 11 more days while in a skilled nursing unit; oral metronidazole, 500 mg every 8 hours, was added to the regimen, which he did complete.
Two weeks after discharge, he visited an outpatient surgeon who ordered CT scans with contrast, the results of which showed a decrease in the size of the abscess to 2.7 × 1.6 cm (Figure 4), with an appearance of abscess as opposed to scar; the peritoneal pus collection had not recurred. The output from the drain had decreased from 25 mL daily at the time of his second hospital discharge to a very scant output at the time of final CT scan. The drain was removed at this point (approximately 6 weeks after initial placement).

Figure 4: CT appearance of the intraparenchymal abscess 5 weeks after drainage and catheter placement plus antibiotic treatment. The size had decreased to 2.7×1.6 cm from the original size of 3.7 × 2.6 cm and had more of an appearance of scar vs infarct.
We made phone contact with the patient after his surgical follow-up visit, and he denied any recurrence of left upper-quadrant pain, fever, severe weakness, or anorexia.
NEXT: Splenic Abscess, Presentation, and more
Splenic Abscess
Splenic abscess is a rare infection that usually is caused by hematogenous spread due to bacteremia (such as caused by endocarditis, pneumonia, cholecystitis, central line infection, or, as in our case, salmonellosis) but can also result from a contiguous infection (such as an adjacent pancreatic abscess). As of 2008, only approximately 600 cases of splenic abscess from all causes had been reported in the worldwide literature.1
The most common pathogens include Klebsiella, Staphylococcus, and Streptococcus species, but anaerobes and fungi also can be causative, especially if the patient is immunocompromised.2-4 The dominant pathogens vary around the world and also can vary with comorbidities. For example, Burkholderia was the most common pathogen in a case series of patients in Singapore, 90% of whom had diabetes.5 Diabetes, endocarditis, HIV infection, chemotherapy, and immunosuppressive drugs after organ transplant greatly increase the risk of splenic abscess formation from any cause. Anatomic disruptions of the spleen from trauma, infarction, or cysts also increase the risk of abscess formation.5
The mortality rate for untreated splenic abscess is 100%6 but decreases to approximately 10% with appropriate treatment.4-10
Splenic abscess due to salmonellosis is uncommon. Two case series of splenic abscesses reported in Asia found that approximately 10% of cases were caused by infection with a Salmonella species.4,5 Splenic abscesses from infection with nontyphoidal Salmonella species are even less common than those due to typhoid fever, probably because nontyphoidal Salmonella is less likely to be an enteroinvasive infection compared with typhoidal Salmonella.
Broadly, human Salmonella infections can be categorized into typhoidal or nontyphoidal gastroenteritis. Salmonella are a genus of gram-negative, motile enteric bacteria in the family Enterobacteriaceae. The type species is Salmonella enterica, which has more than 2500 serovars, with more continuing to be discovered.11 Until 1986, Salmonella had been classified into A, B, C, D, and E subtypes, with B, C, and D types causing most infections in humans, but this has changed to the serovar subspecies classification now used by the World Health Organization. Other Salmonella classification systems also are in use, and this causes confusion among researchers and health professionals.12
Typhoid fever (also known as enteric fever), caused by Salmonella ser Typhi or Parathyphi, is a locally invasive infection of the distal ileum and colon with subsequent systematization that sometimes causes sepsis, extraintestinal manifestations, and even neuropsychiatric manifestations. In comparison, nontyphoidal salmonellosis (caused by such serovars as Typhimurium, Enteritidis, Newport, Javiana, and Heidelberg) is almost always localized to the distal ileum and colon and rarely becomes systemic.12 Immunocompromised persons (such as our patient, who had diabetes and cirrhosis) are more susceptible to bacteremia and subsequent organ abscess. Interestingly, our patient had mild jejunitis on CT scans but no inflammation of the ileum or colon, probably indicating that salmonellosis was resolving.
Nontyphoidal salmonellosis is one of the most common and widely distributed foodborne diseases worldwide, usually acquired through contaminated raw poultry or raw egg yolks, but reptile feces, peanut butter, and contaminated water also have been reported as sources.11,12 The route of acquisition by humans is fecal-oral. Approximately 1.2 million cases of nontyphoidal salmonellosis occur annually in the United States, resulting in approximately 450 deaths.13
Only approximately 5% of persons with Salmonella gastroenteritis will develop bacteremia, with even fewer developing a focal abscess.14 The development of complicated salmonellosis is more likely to occur in patients at the extremes of age and those with immune-altering conditions.5,15 Moreover, certain subtypes of nontyphoidal Salmonella more commonly cause invasive infection (eg, S Enteritidis, S Typhimurium).14 Hepatic and splenic abscesses are the main extraintestinal complications of salmonellosis, with bacteremia also causing endocarditis, pericarditis, cholecystitis, pancreatic abscess, perirectal abscess, cholangitis, subphrenic abscess, adrenal abscess, and spontaneous peritonitis.16 The worldwide incidence of extraintestinal manifestations is 49 per 100,000 cases of nontyphoidal salmonellosis.17
Presentation, Diagnosis, and Treatment
Nontyphoidal salmonellosis causes nausea, vomiting, headache, diarrhea, fever, anorexia, and abdominal cramping lasting approximately 4 to 7 days; for most patients, the condition resolves without treatment.18 Dehydration and electrolyte abnormalities can develop if diarrhea is severe. Rarely, bacteremia can ensue and cause sepsis as well as focal extraintestinal manifestations, which can be difficult to diagnose. Organ abscesses can develop immediately or even years later,14 and they require a high level of suspicion for diagnosis. Patients who develop a splenic abscess most often experience left upper-quadrant pain, fever, and leukocytosis or leukopenia and also can develop left-sided pleural effusion (as noted on chest radiographs) and splenomegaly.19
Our patient had continuous, severe left upper-quadrant pain that initially had prompted ultrasonography and then CT. The diagnostic imaging modality of choice is arguably CT with IV contrast, although ultrasonography can also identify splenic lesions, albeit with less detail.4
Detecting Salmonella promptly in stool, blood, urine, or abscess cultures, then elucidating antibiotic sensitivity in each severe salmonellosis case, is required for a good prognosis, given that resistance to ampicillin, chloramphenicol, and fluoroquinolones is common, especially outside the United States.12 Subtype testing is by way of polymerase chain reaction for nucleic acid detection once adequate culture growth has been achieved20; this is usually done by local health departments. The main purpose of subtyping is for public health, and it is not needed in the treatment of each case. Outbreak detection and isolation is vital to prevent future infections, and all salmonellosis cases must be reported to health departments in the United States.
Treatment of salmonellosis depends on the severity of illness and culture and sensitivity test results. Mild infection (gastroenteritis alone) does not warrant antibiotic treatment.12 Dehydration and/or electrolyte deficiencies should be corrected in all patients. However, antibiotic therapy should be considered for patients at high risk for invasive infection. Furthermore, patients with sepsis, shock, or any extraintestinal manifestations should be hospitalized and promptly started on IV antibiotics. For ill adults, an IV fluoroquinolone plus ceftriaxone should be given empirically while awaiting culture test results.12 For ill children, the empiric regimen is IV azithromycin plus ceftriaxone.12 Antibiotic monotherapy directed by culture and sensitivity test results can then be continued until the infection resolves.
The treatment of splenic abscess traditionally has been with antibiotic therapy along with full or salvage splenectomy; however, over the past 2 decades, successful treatment has been achieved with antibiotic therapy (usually IV) along with CT-guided percutaneous drainage.7-9 Full splenectomy remains the definitive treatment for many patients with splenic abscess but has an average mortality rate of approximately 1.4%.21 Often, the perioperative mortality risk of patients with splenic abscess precludes full splenectomy, such as in patients with unstable vital signs (eg, severe sepsis, septic shock), who have had a recent myocardial infarction, or who have received recent treatment with tissue plasminogen activator. Our patient had cirrhosis and malnutrition and so was determined to have a perioperative risk that precluded open splenectomy. In addition to the risk associated with the operation, full splenectomy puts a patient at greater risk of future encapsulated organism infection, venous thromboembolism, and hematologic malignancies.22,23
The development of CT-guided percutaneous drainage of splenic abscess has expanded the options for treatment of high-risk patients. Gerzof and colleagues24 developed the technique of percutaneous abscess and fluid drainage of intra-abdominal abscesses and described their procedures in 1979. Based on the results of recent case series and case reports, patients who undergo total splenectomy (plus antibiotics) have a higher overall survival rate compared with those who undergo percutaneous drainage (plus antibiotics),4 but we believe that this difference in mortality outcomes is due to selection bias (ie, more severely ill patients are selected for percutaneous drainage, and fewer ill patients are selected for open surgery). Certainly, no randomized controlled trials have been (or could be) performed to confirm the superiority or inferiority of CT-guided splenic abscess drainage over open splenectomy, so no firm conclusion about which technique is best for which patients can be drawn.
Somewhat surprisingly, comparing the outcomes of CT-guided drainage plus antibiotic therapy with the outcomes of long-term antibiotic therapy alone (with no surgical drainage) reveals no clear advantage to CT-guided drainage.4,5,9 Some patients can fully recover after a protracted course of antibiotics alone.16 Nevertheless, surgical drainage is always considered and usually performed. Due to the complexity and severity of splenic abscess, surgical and infectious disease consultations are also required for management. Antibiotic monotherapy is guided by aspiration and/or intra-operative culture and sensitivity test results.
The Take-Home Message
Patients with chronic or critical illness who develop a splenic abscess present an immediate clinical conundrum for the health care team. The extensive treatment course—including long-term antibiotic monotherapy directed at the particular Salmonella serovar, frequent serial imaging, and careful outpatient follow-up—is difficult and does not always ensure a cure. Often, persistent abscess can cause recurrent sepsis once IV antibiotics are stopped, making repeated hospitalization necessary.
Despite these challenges, percutaneous drainage likely has greatly improved the survival of ill and complicated patients who develop splenic abscess, but no randomized controlled trials have proven or disproven this assumption. Once the diagnosis of splenic abscess has been made after CT and/or ultrasonography, delays in treatment must be avoided to ensure a good prognosis. As more people with immune-altering illnesses live longer, the incidence of splenic abscess is likely to increase.
Tara J. Scheufler, DO, is a hospitalist at Good Samaritan Hospital and a clinical assistant professor of emergency medicine at the Boonshoft School of Medicine at Wright State University in Dayton, Ohio.
Angela M. Palitto, DO, is a resident in the Department of Emergency Medicine at the Boonshoft School of Medicine at Wright State University in Dayton, Ohio.
REFERENCES:
- Fotiadis C, Lavranos G, Patapis P, Karatzas G. Abscesses of the spleen: report of three cases. World J Gastroenterol. 2008;14(19):3088-3091.
- Schrier SL. Approach to the adult with splenomegaly and other splenic disorders. UpToDate. https://www.uptodate.com/contents/approach-to-the-adult-with-splenomegaly-and-other-splenic-disorders. Updated July 14, 2017. Accessed August 18, 2017.
- Krzysztof Ł, Krysiak R, Basiak M, et al. Diagnostic difficulties in diagnosis of splenic abscesses [in Polish]. Wiad Lek. 2007;60(1-2):83-86.
- Chang K-C, Chuah S-K, Changchien C-S, et al. Clinical characteristics and prognostic factors of splenic abscess: a review of 67 cases in a single medical center of Taiwan. World J Gastroenterol. 2006;12(3):460-464.
- Ng CY, Leong EC, Chng HC. Ten-year series of splenic abscesses in a general hospital in Singapore. Ann Acad Med Singapore. 2008;37(9):749-752.
- Simson JN. Solitary abscess of the spleen. Br J Surg. 1980;67(2):106-110.
- Westh H, Reines E, Skibsted L. Splenic abscesses: a review of 20 cases. Scand J Infect Dis. 1990;22(5):569-573.
- Zerem E, Bergsland J. Ultrasound guided percutaneous treatment for splenic abscesses: the significance in treatment of critically ill patients. World J Gastroenterol. 2006;12(45):7341-7345.
- Liu KY, Shyr YM, Su CH, Wu CW, Lee LY, Lui WY. Splenic abscess—a changing trend in treatment. S Afr J Surg. 2000;38(3):55-57.
- Nasr B, Chhaider A, Mabrouk MB, et al. Diagnosis and treatment of splenic abscess (about 11 cases). J Gastroenterol Hepatol Res. 2013;2(9):786-790.
- Salmonella (non-typhoidal). World Health Organization. https://www.who.int/mediacentre/factsheets/fs139/en. Updated December 2016. Accessed August 18, 2017.
- Klochko A, Wallace MR. Salmonellosis. Medscape. http://emedicinemedscape.com/article/228174-overview. Updated October 21, 2016. Accessed August 18, 2017.
- Voetsch AC, Van Gilder TJ, Angulo FJ, et al; Emerging Infections Program FoodNet Working Group. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis. 2004;38(suppl 3):S127-S134.
- Çabadak H, Erbay A, Karaman K, Şen S, Tezer-Tekçe Y. Splenic abscess due to Salmonella enteritidis. Infect Dis Rep. 2012;4(1):e4.
- Ghidirim G, Rojnoveanu G, Mişin I, Gagauz I, Gurghiş R. Splenic abscess—etiologic, clinical and diagnostic features [in Romanian]. Chirurgia (Bucur). 2007;102(3):309-314.
- Cohen JI, Bartlett JA, Corey GR. Extra-intestinal manifestations of Salmonella infections. Medicine (Baltimore). 1987;66(5):349-388.
- Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal Salmonella disease, 2010. Emerg Infect Dis. 2015;21(6):941-949.
- Salmonella. Centers for Disease Control and Prevention. https://www.cdc.gov/salmonella. Updated August 18, 2017. Accessed August 18, 2017.
- Liu Y-H, Liu C-P, Lee C-M. Splenic abscesses at a tertiary medical center in northern Taiwan. J Microbiol Immunol Infect. 2014;47(2):104-108.
- Association of Public Health Laboratories. Salmonella Serotyping in US Public Health Laboratories. Silver Spring, MD: Association of Public Health Laboratories; November 2014. https://www.aphl.org/aboutAPHL/publications/Documents/FS_SalmonellaSustainabilityWhitePaper_Nov2014.pdf. Accessed August 18, 2017.
- Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect. 2001;43(3):182-186.
- Kristinsson SY, Gridley G, Hoover RN, Check D, Landgren O. Long-term risks after splenectomy among 8,149 cancer-free American veterans: a cohort study with up to 27 years follow-up. Haematologica. 2014;99(2):392-398.
- Pachter HL, Grau J. The current status of splenic preservation. Adv Surg. 2000;34:137-174.
- Gerzof SG, Robbins AH, Birkett DH, Johnson WC, Pugatch RD, Vincent ME. Percutaneous catheter drainage of abdominal abscesses guided by ultrasound and computed tomography. AJR Am J Roentgenol. 1979;133(1):1-8.


