Preoperative Evaluation and Care: The Cardiac Patient Undergoes Noncardiac Surgery
ABSTRACT: Cardiac patients that require surgery may be particularly challenging in perioperative management. Vigilance for the prevention of acute complication is critical. Physiologic factors associated with surgery predispose patients with underlying coronary disease to increased morbidity and mortality. It is therefore up to the clinician to integrate information from the history, physical examination, and ECG to develop an initial estimate of perioperative cardiac risk and to determine the role of anticoagulation in the treatment.
The preoperative evaluation is an important interaction between the patient and the physician. This evaluation enables the surgeon to carefully assess the patient’s medical conditions and overall health status. It may also be used to educate the patient on the risks and benefits of the proposed surgery, review alternative options, and address possible complications during the perioperative period. The additional time invested in a preoperative evaluation yields an improved patient–physician relationship and reduces surgical complications.1
This article will offer guidelines for performing a complete preoperative evaluation, as well as highlight specific issues and management problems that arise in cardiac patients undergoing noncardiac surgery.
Risks and Complications
Cardiovascular complications pose significant risks to patients undergoing major noncardiac surgery. A 1977 prospective study that evaluated 1001 coronary artery disease (CAD) patients over age 40 found that the frequency of significant postoperative cardiac complications (ie, nonfatal myocardial infarction [MI], pulmonary edema, or ventricular tachycardia) or death was 5.8 %.2
Patients with underlying cardiovascular disease (ie, peripheral artery disease [PAD] or stroke [CVA]) have an increased risk of perioperative cardiac complications and a higher incidence of significant CAD.3,4 In addition, this population reports a 5 times higher rate of left ventricular systolic dysfunction—left ventricular ejection fraction less or equal than 40%.5
Physiologic factors associated with surgery predispose CAD patients to myocardial ischemia. These include blood loss, volume shifts, enhanced myocardial oxygen demand from changes in heart rate and blood pressure, as well as an increase in postoperative platelet reactivity.6
Initial Preoperative Evaluation
A detailed history and assessment of exercise tolerance, along with physical examination, and a resting ECG are used for an initial estimate of perioperative cardiac risk. Specifically, the initial preoperative evaluation should include a detailed history inquiring about previous coronary heart disease, symptoms of angina, heart failure (CHF), aortic stenosis (AS), severe hypertension, and PAD. ECG may yield valuable information for risk assessment. Clinical predicators are listed in Algorithm-1.
• Functional capacity. This assessment of cardiac functional status can provide valuable prognostic information. Patients with good functional status have a lower risk of complications.2 Functional status is expressed in metabolic equivalents: 1 MET is defined as 3.5 mL O2 uptake/kg per min, which is the resting oxygen uptake in a sitting position. Perioperative cardiac and long-term risk is increased in patients unable to meet a 4-MET demand during most normal daily activities.
Various activity scales provide the clinician with a set of questions to determine a patient's functional capacity.7 Indicators of functional status include the following:
• Can care for one’s self—ie, eating, dressing, or using the toilet (1 MET).
• Can walk up a flight of steps or a hill (4 METs).
• Does heavy work around the house such as scrubbing floors, lifting, or moving heavy furniture (4-10 METs).
• Participates in strenuous sports such as swimming, singles tennis, football, basketball, and skiing (>10 METs).
• Physical examination. This should include blood pressure measurements in both arms, analysis of carotid artery and jugular venous pulsations for the quality of the pulse contour, and the presence of bruits, auscultation of the lungs, precordial palpation and auscultation, abdominal palpation, and examination of the extremities for edema and vascular integrity. Important findings include evidence of HF or a murmur suspicious for AS since poorly-controlled HF and significant AS increase perioperative risk (Algorithm-1).
Significant ST segment elevation or depression have been associated with an increased incidence of perioperative cardiac complications.8,9 The 2007 American College of Cardiology (ACC)/ American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation (unchanged in the 2009 update) recommended a preoperative resting 12-lead ECG in patients with at least one clinical risk factor scheduled to undergo vascular surgery (Algorithm-1) or those patients scheduled to undergo intermediate-risk surgery with known cardiovascular disease, PAD, or cerebrovascular disease.10
According to a preoperative assessement of risk using the revised risk index (Algorithm-1), in-hospital mortality rates in patients not treated with beta blockers increased progressively from 1.4% to 7.4%.10
• Stress testing. Recommendations for preoperative stress testing are based upon clinical risk factors, functional capacity and the surgery-specific risk. The 2007 ACC/AHA guidelines concluded that the evidence was in favor of benefit of preoperative stress testing for:10
o Patients at high risk (≥3 revised cardiac index criteria) and with poor functional capacity (<4 METS) who are scheduled for vascular surgery when such testing will change management.
The evidence was considered less well established for the benefit of preoperative stress testing in the following circumstances:
o Patients with at least 1 clinical risk factor and poor functional capacity (<4 METS) who are scheduled for intermediate-risk surgery when such testing will change management.
o Patients with at least 1 clinical risk factor and good functional capacity (≥4 METS) who are scheduled for vascular surgery.
• Resting echocardiography. We suggest resting echocardiography to quantify valvular dysfunction in patients with a murmur, to evaluate ventricular dysfunction in poorly controlled HF, or in dyspnea of uncertain cause or to evaluate for possible pulmonary hypertension.11 Routine echocardiography is not recommended.10,11
• Preoperative revascularization. The proportion of noncardiac surgery patients who undergo preoperative cardiac catheterization and revascularization appears to be very low.12 In 3 studies evaluating the ACC/AHA guidelines, 2% to 11% of patients underwent coronary angiography and only 0% to 2% underwent preoperative revascularization.13-15 These findings indicate that there are few asymptomatic intermediate-risk patients who are candidates for preoperative revascularization.
Obesity
The prevalence of CHD and its risk factors is increased in obese patients. A 2009 AHA scientific advisory on obesity noted an association with cardiac and pulmonary diseases, which may negatively affect the outcome of surgery.16 For these reasons, obese patients are at increased risk for adverse cardiovascular events at the time of noncardiac surgery.
However, the estimation of risk is more difficult in obese patients due to the undetermined significance of certain components of the history (dyspnea) and physical examination (lower-extremity edema). The generally poor exercise capacity of the obese further complicates risk assessment.
The issue of whether the preoperative approach to obese patients should differ from that in the general population is uncertain. This AHA 2009 advisory on severely obese patients undergoing surgery states that specific tests should be performed only if the results will change management.16 The following specific suggestions are offered:
• A 12-lead ECG is reasonable in all obese patients with at least 1 risk factor for CHD (diabetes, smoking, hypertension, or hyperlipidemia) or poor exercise tolerance.
• A chest radiograph (posteroanterior and lateral) should be obtained on all severely obese patients prior to surgery, as the sensitivity and specificity (for the diagnosis of cardiopulmonary disease) of the physical examination is decreased.
Management
Patients with ACTIVE cardiac conditions (eg, unstable coronary syndromes, decompensated HF, significant arrhythmias, or severe valvular stenosis) not undergoing emergency surgery should first be managed, according to Algorithm-1:
o Patients without active cardiac symptoms undergoing low risk surgery (Algorithm-1) or those with good functional capacity (MET level ≥4) require no preoperative testing aside from an ECG.
o Patients with poor or unknown functional capacity, or potential cardiac symptoms who are scheduled to undergo intermediate risk or vascular surgery are managed according to the number of clinical risk factors (Algorithm-1). For such patients, heart rate control with beta blockade may be of benefit.
o In patients with 1 or 2 clinical risk factors, it is reasonable to proceed to surgery unless testing will alter management.
o In patients with 3 of more clinical risk factors scheduled to undergo intermediate risk surgery (Algorithm-1), it is reasonable to proceed without noninvasive testing unless that testing will alter management.
o In patients with 3 or more clinical risk factors scheduled to undergo vascular surgery, the guidelines suggest noninvasive testing if it will alter management.
• Elective surgery. In this setting there is adequate time to complete recommended tests and if necessary, revascularization procedures. If preoperative PCI is considered, any potential benefit must be balanced against the requirements for a full course of aggressive antiplatelet therapy with aspirin and clopidogrel, generally 3 to 6 months. Premature discontinuation of antiplatelet therapy carries a substantial risk of stent thrombosis, a risk that may be increased further by surgery.17
• Urgent surgery. For urgent surgery (eg, required during the same admission but a delay of days may be acceptable), initial risk estimates should be made. However, the value of additional testing and treatment is often limited except for identifying and stabilizing patients with unstable cardiac disease.12
• Emergent surgery. Emergent surgery carries unique, often substantial risks. In these cases, risk indices derived from elective surgery cohorts are not accurate although they may provide an estimate of the minimal risk. Further testing and interventions are unlikely to be beneficial. Despite the elevated risk, patients are usually best served by proceeding directly to surgery. Beta blockers may be helpful in patients who are hemodynamically stable and in whom benefit has been shown.
Perioperative initiation of beta blocker is recommended in patients with at least one cardiac risk factor who are undergoing vascular surgery.18 If the surgical approach would be altered on the basis of additional risk stratification (ie, surgery would be delayed, canceled, or performed on dual antiplatelet therapy), additional testing and treatment may be warranted.

Figure 1. Algorithm based on the AHA/ACC guidelines for perioperative cardiovascular risk stratification.10
The Role of Anticoagulants
The role for anticoagulants in many cardiovascular disorders is well established and their use as prophylaxis against stroke or thromboembolism is increasing. Although continuation of anticoagulation increases the risk of bleeding following invasive procedures, interruption of such therapy may increase the risk of thromboembolism in patients taking anti coagulants to prevent thrombosis.15,19,20
The risk of under anticoagulation varies with the type of thromboembolic event. While recurrent deep vein thrombosis (DVT) or pulmonary embolism (PE) is associated with an approximately 5% risk of fatal PE,21 the consequences of arterial thromboembolism from atrial fibrillation (AF) or prosthetic heart valves are much more serious—with 20% being fatal and 40% causing permanent disability. Figure 2 has been formulated in order to estimate this risk (Algorithm-2).22,23-27
Appropriate use of alternative strategies, such as intravenous heparin or subcutaneous low molecular weight heparin (LMWH) to provide antithrombotic coverage (ie, "bridging" anticoagulation) during the period when warfarin is withdrawn or reintroduced have been used in an attempt to minimize risks. The 2 most common clinical settings—chronic AF and prosthetic heart valves—are discussed below. Risk is reduced by two-thirds with warfarin use.
• Arterial thromboembolism is most commonly associated with AF. Embolic stroke is fatal or associated with a severe neurologic deficit in over 60% of AF
patients.23,24
Patients with AF not due to valvular heart disease have an overall risk of systemic embolism of 4% to 5% per year in the absence of warfarin therapy. Anticoagulation reduces the risk of embolization by approximately two-thirds in this
setting.25,28
• Systemic embolization (predominantly cerebrovascular events) occurs approximately 0.7% to 1% per patient per year with mechanical valves who are treated with warfarin, 2.2% per patient per year with aspirin, and 4% with no anticoagulation. Within this group, those with mitral valve prostheses are at approximately twice the risk compared to those with aortic valve prostheses.29 A major advantage of the bioprosthetic valve is freedom from anticoagulation after 3 months of treatment and low risk for systemic embolism thereafter.30 Other causes of thromboembolism include a dilated and poorly contractile left ventricle or a left ventricular aneurysm in which intraventricular thrombi may form and embolize.31-33
Risk of Bleeding
The risk of bleeding that occurs when patients taking anticoagulant therapy are in surgery varies and is dependent upon age, presence of other disease states (ie, chronic renal disease), type of surgery or procedure34, anticoagulant regimen (intensity and duration), the use of other drugs that affect hemostasis (ie, aspirin or other antiplatelet agents), the stability of anticoagulation, and the degree of anticoagulation.15,35,36
The type of surgery or procedure—prolonged, complex, and major surgery—is much more likely to cause significant bleeding problems than short, simple, and minor surgical procedures. For example:
• Low bleeding risk procedures. Most patients can undergo low risk surgical procedures (ie, cataract surgery, coronary arteriography, venography, joint aspiration, dental procedures such as tooth extraction and root canal, minor skin procedures, arthrocentesis, bone marrow biopsy) without alteration of their anticoagulation regimen.37-39 In such patients, oral anticoagulation with a vitamin K antagonist can be continued at or below the low end of the therapeutic range (eg, INR 1.7 to 2.3).
• High bleeding risk procedures. Complex or high risk surgical procedures (eg, open-heart surgery, abdominal vascular surgery, intracranial or spinal surgery, major cancer surgery, urologic procedures) require discontinuation of oral anticoagulation, followed by temporary perioperative coverage with unfractionated heparin or LMWH in those patients who are at high risk of thromboembolism.40
Warfarin
After stopping warfarin, it usually takes 2 to 3 days for the INR to fall to below 2.0, and 4 to 6 days for the INR to normalize. Once the INR is 1.5 or below, surgery can be performed with relative safety in most cases, although a normalized INR is typically required in patients undergoing surgery associated with a high bleeding risk (eg, intracranial, spinal, urologic) or if spinal anesthesia is to be used following surgery.
After warfarin is restarted, it takes approximately 5 days for the INR to rise above 2.0. Therefore, it is estimated that if warfarin is withheld for 5 days before surgery and is restarted as soon as possible afterwards, patients would have a subtherapeutic INR for approximately 4 days before surgery and 4 days after surgery.41
Vitamin K Antagonists
Reversing the anticoagulant activity of warfarin and other vitamin K antagonists depends upon the amount of time available before the surgical or invasive procedure and the elimination half-life of the vitamin K antagonist—ie, 36 to 42 hours for warfarin, 8 to 12 hours for acenocoumarol, and 96 to 140 hours for phenprocoumon—as well as the estimated bleeding and thrombotic risk.
• Fully elective surgery. In patients with an INR between 2.0 and 3.0 who are undergoing elective surgery that requires temporary cessation of anticoagulation, warfarin should be withheld for approximately 5 days to allow the INR to be normal (INR <1.3) or near normal (INR 1.3 to 1.4) before surgery.41-43
• Semi-urgent surgery. If more rapid reversal (1-2 days) of warfarin anticoagulation is required, warfarin should be withheld and a small dose of vitamin K administered, either intravenously (1.0 to 2.5 mg) or orally (2.5 to 5.0 mg).
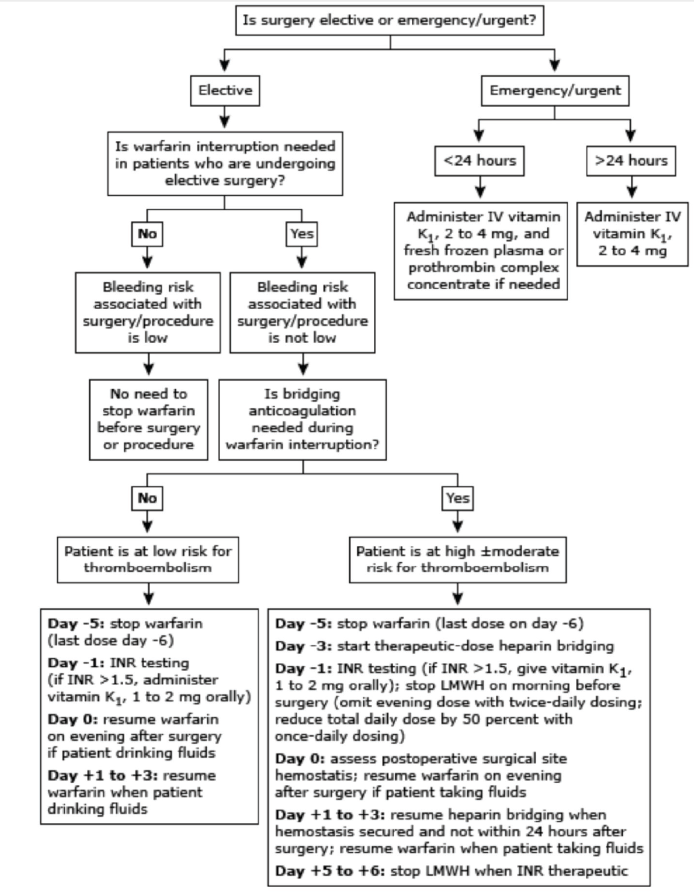
Figure 2. Overview of perioperative management of warfarin therapy and heparin bridging before and after surgery/procedure.33
Bridging Anticoagulation
Bridging anticoagulation can be defined as the administration of a short-acting anticoagulant, typically a LMWH, during the perioperative interruption of warfarin. The goal of bridging is to minimize the time the patient is not being anticoagulated, thereby minimizing the patient’s risk for perioperative thromboembolism.
Anticoagulant drugs and doses used for bridging anticoagulation include:
• Unfractionated heparin. The biologic half-life of intravenous unfractionated heparin (UFH) is approximately 45 minutes.27,44 Thus, most bridging anticoagulation studies have suggested that intravenous UFH should be stopped 4 to 5 hours before the planned surgery or procedure—a time interval that is approximately 5 elimination half-lives of UFH.
If UFH is used for bridging, it is administered by an infusion to attain an activated partial thromboplastin time (aPTT) that is 1.5 to 2 times the control aPTT, in a manner similar to that used to treat patients with acute venous thromboembolism.
• LMWH. The biologic half-life of subcutaneous LMWH is approximately 3 to 5 hours.27,44 Thus, most bridging anticoagulation guidelines suggest that the last dose of subcutaneous LMWH should be given 24 hours before the planned surgery or procedure, a time interval that is approximately 5 elimination half-lives of LMWH.
If LMWH is used for bridging, it can be given as a therapeutic-dose regimen, either twice-daily (eg, enoxaparin dosed 1 mg/kg twice daily) or once-daily (eg, dalteparin dosed 200 international units/kg once daily). LMWH can also be given at a lower dose, referred to as an intermediate-dose regimen (eg, enoxaparin, 40 mg twice daily), which is higher than the dose typically administered for postoperative prevention of DVT (eg, enoxaparin, 40 mg once daily). There is no established dosing regimen, although therapeutic-dose regimens tend to be used more often in North America.
• Synthetic heparin derivative. Fondaparinux is a synthetic pentascharide that is chemically similar to heparin. It is factor Xa inhibitor. It is completely bioavailable with subcutaneously injection. Its half-life is 17 hours and routine monitoring is not required. Fondaparinux does not have an antidote; however, factor VIIa has anecdotally been reported to help control bleeding.45,46 Idraparinux is very similar to fondaparinux, but has a much longer half-life of 80 hours, thus can be subcutaneously on a weekly basis.45
• Direct thrombin inhibitors. These include:
o Argatroban. This small peptide molecule competitively binds to thrombin and has a half-life 40 to 50 minutes. It can be administered only by the intravenous route and achieves a steady state between 1 and 3 hours. Dose adjustment may be required in patient with hepatic failure.47
o Lepirudin. This direct thrombin inhibitor with a half-life of 1.3 hours and can be administered only through intravenous route, it is cleared renally.48
o Bivalirudin. This direct thrombin inhibitor is also administered through the intravenous route and has a half-life of 25 minutes. About 80% is cleared by proteolysis and 20% by renal filtration.49
• Newer orally-active anticoagulants. There are two novel groups of oral anticoagulants: factor Xa inhibitors (rivaroxaban, apixaban) and direct thrombin inhibitors (dabigatran). They are fixed-dose oral agents that, compared to vitamin K antagonists, do not require routine laboratory monitoring and dose adjustments. They are also less susceptible to dietary and drug interaction. Additionally, these agents reach their peak efficacy within 1 to 4 hours after ingestion; therefore, a prolonged period of bridging therapy is not required when switching from initial treatment (eg, with UFH or LMWH) to these agents.
Disadvantages include twice-daily dosing (dabigatran, apixaban), higher cost, lack of an antidote/reversing agent, the potential need for dose adjustment with chronic kidney disease, and lack of long term safety and “real-world” data.50
The abovementioned outline and algorithm helps practitioners make a careful assessment of the perioperative cardiac patient. Remember, anticoagulation is an important aspect in the management of surgical patients and is continuing to evolve. ■
References
1.Schwartz R, Maneckshana, B, Rauscher G, et al. Preoperative evaluation in management. Medline. 2013 May 15. Available at: http://emedicine.medscape.com/article/1127055-overview.
2.Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845-850.
3.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
4.Wong T, Detsky AS. Preoperative cardiac risk assessment for patients having peripheral vascular surgery. Ann Intern Med. 1992; 116:743-753.
5.Kelly R, Staines A, MacWalter R, et al. The prevalence of treatable left ventricular systolic dysfunction in patients who present with noncardiac vascular episodes: a case-control study. J Am Coll Cardiol. 2002;39:219-224.
6.Stampfer MJ, Grodstein F, Bechtel S. Postmenopausal estrogen and cardiovascular disease. Contemp Intern Med. 1994;6:47-56,59.
7.Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery-exective summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (commity to update the 1996 guidelines on preoperative cardiovascular evaluation for non cardiac surgery). J Am Coll Cardiol. 2002;29:542.
8. Eagle KA, Coley CM, Newell JB, et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Anh Intern Med. 1989;110:859-866.
9. Baron JF, Mundler O, Bertrand M, et al. Dipyridamole-thallium scintigraphy and gated radionuclide angiography to assess cardiac risk before abdominal aortic surgery. N Engl J Med. 1994;330:663-669
10.Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:e169.
11.Auerbach A, Goldman L. Assessing and reducing the cardiac risk of noncardiac surgery. Circulation. 2006;113:1361-1376.
12.Wesorick DH, Eagle KA. The preoperative cardiovascular evaluation of the intermediate-risk patient: new data, changing strategies. Am J Med. 2005;118:1413.
13.Bartels C, Bechtel JF, Hossmann V, Horsch S. Cardiac risk stratification for high-risk vascular surgery. Circulation. 1997;95:2473-2475.
14.Froehlich JB, Karavite D, Russman PL, et al. American College of Cardiology/American Heart Association preoperative assessment guidelines reduce resource utilization before aortic surgery. J Vasc Surg. 2002;36:758-763.
15.Almanaseer Y, Mukherjee D, Kline-Rogers EM, et al. Implementation of the ACC/AHA guidelines for preoperative cardiac risk assessment in a general medicine preoperative clinic: improving efficiency and preserving outcomes. Cardiology 2005:103:24.682.
16.Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation. 2009;120:86-95.
17.Shammash J, Kimmel S, Morgan J. Estimation of cardiac risk prior to non cardiac surgery. UpToDate. 2012 Jul 23. Available at: http://www.uptodate.com/contents/estimation-of-cardiac-risk-prior-to-noncardiac-surgery.
18.Stone JG, Foex P, Sear JW, et al. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of beta-adrenergic blocking agent. Anesthesiology. 1988;68:495-500.
19.Kakkar VV, Cohen AT, Edmonson RA, et al. Low molecular weight versus standard heparin for prevention of venous thromboembolism after major abdominal surgery. The Thromboprophylaxis Collaborative Group. Lancet. 1993;341:259-265.
20.Jaffer AK. Perioperative management of warfarin and antiplatelet therapy. Cleve Clin J Med. 2009;76 Suppl 4:S37-44.
21.Douketis JD, Gu CS, Schulman S, et al. The risk for fatal pulmonary embolism after discontinuing anticoagulant therapy for venous thromboembolism. Ann Intern Med. 2007;147:766-774.
22.Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954-2962.
23.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760-1764.
24.Jørgensen HS, Nakayama H, Reith J, et al. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke. 1996;27:1765-1769.
25.Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest. 2008;133:546S.
26.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449-1457.
27.Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S-350S. .
28.Lip GY, Lowe GD. ABC of atrial fibrillation. Antithrombotic treatment for atrial fibrillation. BMJ. 1996;312:45-49.
29.Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994;89:635.
30.Whitlock RP, Sun JC, Fremes SE, et al. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e576S-600S.
31.Lip GY. Intracardiac thrombus formation in cardiac impairment: the role of anticoagulant therapy. Postgrad Med J. 1996;72:731-738.
32.Loh E, Sutton MS, Wun CC, et al. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med. 1997;336:251-257.
33.Nixon JV. Left ventricular mural thrombus. Arch Intern Med. 1983;143:1567-1571.
34.Otley CC. Continuation of medically necessary aspirin and warfarin during cutaneous surgery. Mayo Clin Proc. 2003;78:1392-1396.
35.Nieuwenhuis HK, Albada J, Banga JD, Sixma JJ. Identification of risk factors for bleeding during treatment of acute venous thromboembolism with heparin or low molecular weight heparin. Blood. 1991;78:2337-2343.
36.Levine MN, Raskob G, Landefeld S, Hirsh J. Hemorrhagic complications of anticoagulant treatment. Chest. 1995;108:276S-290S.
37.Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med. 2003;163:901-908.
38.De Caterina R, Husted S, Wallentin L, et al. Anticoagulants in heart disease: current status and perspectives. Eur Heart J. 2007;28:880-913.
39.Wysokinski WE, McBane RD 2nd. Periprocedural bridging management of anticoagulation. Circulation. 2012;126:486-490.
40.Wysokinski WE, McBane RD, Daniels PR, et al. Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation. Mayo Clin Proc. 2008;83:639-645.
41.Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997;336:1506.
42.White RH, McKittrick T, Hutchinson R, Twitchell J. Temporary discontinuation of warfarin therapy: changes in the international normalized ratio. Ann Intern Med. 1995;122:40-42.
43.Larson BJ, Zumberg MS, Kitchens CS. A feasibility study of continuing dose-reduced warfarin for invasive procedures in patients with high thromboembolic risk. Chest. 2005;127:922-927.
44.Hirsh J, Bauer KA, Donati MB, et al. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest. 2008;133:141S-159S.
45.Crowther M, Weitz JI. New anticoagulants: an update. Clin Adv Hematol Oncol. 2004;2:743-749.
46.Turpie AG, Eriksson Bl, Lassen MR, Bauer KA. Fondaparinux. The first selective factor Xa inhibitor. Curr Opin Hematol. 2003;10;327-332.
47.Yeh RW, Jang IK. Argatroban: update. Am Heart J. 2006;151:1131-1138.
48.Nutescu EA, Shapiro NL, Chevalier A. New anticoagulant agents: direct thrombin inhibitors. Cardiol Clin. 2008;26:169-187.
49.Warkentin TE, Greinacher A, Koster A. Bivalirudin. Thromb Haemost. 2008;99:830-839.
50.Gage BF. Can we rely on RE-LY? N Engl J Med. 2009;361:1200.
Abdul Ahad Qazizada, MD, is a family medicine physician at Family Medicine Clinic, Naval Hospital, and a hospitalist at Orange Park Hospital in Jacksonville, FL.
James C. Higgins, CAPT, MC, USN, Ret., is a faculty family physician in the family medicine residency program at the Naval Hospital Jacksonville, FL, and assistant clinical professor of family medicine at the Uniformed Services University of the Healthy Services F. Edward Hebert School of Medicine, Bethesda, MD.


