Evaluating Patients With Cognitive Changes: A New Imaging Option
ABSTRACT: Alzheimer’s disease (AD) is a neurodegenerative disease characterized by progressive and irreversible cognitive decline, primarily affecting the aging population. The disease is also associated with a substantial economic burden. Currently, there are 3 FDA-approved diagnostic drugs visualizing beta-amyloid on a positron emission tomography scan of the brain to evaluate patients for AD and other causes of cognitive decline. This article reviews the prevalence, cost, and diagnosis of AD and presents 2 case studies demonstrating the clinical utility of florbetapir, an amyloid imaging compound, as part of the diagnostic evaluation in patients presenting with cognitive impairment.
Alzheimer’s disease (AD), the most common form of dementia, is a neurodegenerative disease characterized by progressive and irreversible cognitive decline.1,2 The pathological hallmarks of AD are the presence of beta-amyloid neuritic plaques and neurofibullary tangles comprised of hyperphosphylated tau protein.1
Evidence suggests that these 2 structures are related to the neuronal injury and brain atrophy that is seen in patients with AD. The presence of both of these structures is required for the pathological confirmation of AD—traditionally only identified by brain biopsy or autopsy.
The current understanding of the disease is that the amyloid plaques begin accumulating up to 15 to 20 years before clinical symptoms are evident.3 The most prominent clinical feature of AD is an early impairment of episodic memory, which manifests as short-term memory loss, unusual repeated omissions, and difficulty learning new information.2 At this point, there is already widespread deposition of beta-amyloid neuritic plaques within the cortical strip, or grey matter.
According to 2014 data from the Alzheimer’s Association, an estimated 5.4 million people have AD; it is the sixth leading cause of death in the United States. Age is the primary risk for AD, with 1 in 8 people age 65 and older battling the disease. By age 85, the pathologic prevalence increases up to 45%. The number of Americans with AD is expected to increase significantly in the coming years as the baby boom generation ages. By 2050, the prevalence of AD for people age 65 and older is projected to triple.4 The US population is aging, and by the year 2030, people age 65 and older will make up 19% of the population.5
There are no FDA-approved treatments addressing the pathology of AD on the market today. Treatments are limited to symptomatic therapies without disease modification. Keep in mind that the Alzheimer’s Association listed AD as one of the most expensive diseases in the United States. In 2014, the direct costs associated with caring for patients with AD was estimated at $214 billion, including $150 billion in costs to Medicare and Medicaid. The total cost of caring for this patient population is projected to increase to $1.2 trillion in 2050.4
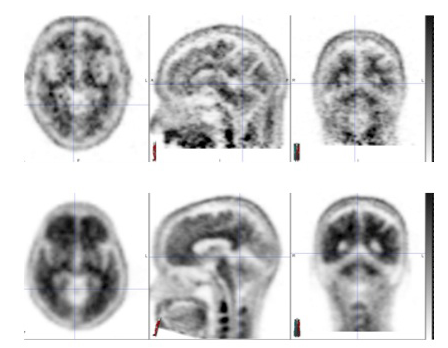
Figure 1. Amyvid PET scans: negative images (top row) and positive images (bottom row).
A Difficult Diagnosis
Efforts are ongoing to better define the pathophysiology of AD, which will hopefully lead to disease-modifying treatments in contrast to the currently available symptomatic treatments (with variable and limited efficacy). The first step is to develop better diagnostic tools to ensure an accurate clinical diagnosis. Wollman and Prohovnik6 reviewed the literature to assess for the sensitivity and specificity of clinical diagnosis versus structural (CT and MRI) and functional (single photon emission computed tomography and metabolic positron emission tomography [PET]) neuroimaging when evaluating a patient with cognitive deficits. Overall, the findings showed that the sensitivity for clinical diagnosis ranges from 39% to 98% and specificity ranges from 33% to 100%. The variability in specificity of the clinical diagnosis is dependent on the experience, expertise, and skill of the clinician. The results indicated a wide range of sensitivity and specificity with the neuroimaging techniques, like with clinical diagnosis. The literature review suggested that sensitivity and specificity improved when combining clinical diagnosis with neuroimaging.6
The use of biomarkers is becoming increasingly important and plays a large role in the new diagnostic criteria for cognitive changes presumed related to AD pathology.7 Cerebrospinal fluid (CSF) biomarkers have been looked at for many years though are not yet standardized and results can have large variability from lab to lab.8 After data were collected from 84 laboratories through a quality control study from the Alzheimer’s Association, the variability was felt to be too high to consider the testing reliable.8 Previous reviews also documented variability between testing kits.9 In our clinical experience with CSF analysis for AD biomarkers, there have been many circumstances when the analysis is inconclusive. For example, a low beta-amyloid but normal phosphorylated tau, or vice versa.
PET Imaging in AD Workup
Currently, there are 3 radioligand tracers FDA-approved to identify the presence or absence of amyloid plaque in the cortical strip, with only one currently commercially available. The amyloid stain is tagged to an flourine-18 radiotracer and detected by noninvasive PET imaging. The first tracer FDA-approved was florbetapir (Amyvid) in April 2012. Florbetapir has a sensitivity of 96%, and specificity of 100% in detecting amyloid in the cortical strip.10 This was followed by the FDA approvals of flutematamol (Vizamyl) in November 2013 and florbetaben (Neuraceq) in March 2014. These tracers have a longer half-life, for example 110 minutes for florebetapir, then the previously utilized tracer Pittsburgh Compound B (PiB) which is 20 minutes. With a longer half-life, clinical use is more practical. PiB is only practical for research since the tracer had to be prepared on site.11
Unlike the CSF biomarkers, the florbetapir scan is interpreted as a binary read, (eg, positive or negative for excess amyloid protein in the cortical strip). A positive scan alone does not confirm AD, but adds to the diagnostic certainty in the experienced clinician’s evaluation. An amyloid labeling PET scan should only be considered in patients undergoing an evaluation for memory loss or other cognitive complaints, and not in asymptomatic individuals.
There have been questions about how these tracers would be used in the evaluation of patients with cognitive impairments. Concerns about how the test results would be applied for management decisions and improve patient outcomes have been raised; these are valid concerns considering there are no FDA-approved disease-modifying treatments once the clinical diagnosis is established. Furthermore, these concerns are pertinent in this era of limited financial resources, and in fact, the 3 FDA-approved tracers are not currently reimbursed for clinical practice under the Centers for Medicare and Medicaid guidelines.12 The cost of the scan varies depending on location.
Workup for Cognitive Impairment
Our clinic, which specializes in cognitive disorders, used the florbetapir scan over the last 2 years in patients who were being evaluated for cognitive impairment; to date, the florbetapir scan has been used to evaluate over 100 cases.
Patients undergo complete neurologic examination, including detailed history as well as physical examination. The examination also includes structural imaging either with MRI or CT, multiple laboratory screens, and cognitive testing. The extent of cognitive testing is based on the patient’s presentation. The possible causes of cognitive impairment, as well as an outline of recommended diagnostic studies, are reviewed with the patient and family/caregivers. The amyloid PET scan is then considered when it is felt that the scan could add diagnostic certainty to the clinical diagnosis.
A detailed discussion about the limitations of the study, including the possibility of false negatives, increase incidence of amyloidopathy with aging, and nonspecificity (eg, positivity in other degenerative diseases such as Lewy body dementia and inclusion body myositis) is recommended. Based on the results of the study, various management options are outlined.
If the test results are positive, it suggests that the patient’s cognitive impairments is on the basis of AD pathology. Patients are reminded that there is no cure or disease-modifying treatment available at this time.
Note: In our experience, we have yet to have a patient or family/caregiver decline proceeding with the study after this discussion.
(Case studies on next page)
Case Studies
The following case studies demonstrate the clinical utility of the florbetapir PET scan as part of the diagnostic evaluation.
Case Study #1: Patient With Mild Language Impairments
A 70-year-old male consulted the clinic for symptoms of mild language impairments beginning 1 year prior to his initial consultation. He is a retired judge and the impairments were mild, so he was able to continue working as a law professor. Function was not affected as he continued daily activities and managed his home and finances.
Physical examination. At his initial office visit, a thorough history was first collected. His medical history was unremarkable and a physical examination was normal. His Mini-Mental State Examination (MMSE) score was 28/30 with intact memory encoding and recall. His speech was fluent.
Laboratory tests. His initial diagnostic workup included a brain MRI without contrast and bloodwork. Typical laboratory workup includes a complete metabolic panel, complete blood count, thyroid studies, autoimmune screening, coagulation studies, syphilis screen, vitamin levels, and homocysteine. His workup was unremarkable.
Diagnosis. Based on his clinical history, workup, and exam, the suspected diagnosis was a non-AD pathology primary progressive aphasia (PPA)versus logopenic PPA, which is frequently associated with AD pathology. The difference between these 2 etiologies is that clinically suggested PPA is commonly associated with tau or TDP-43 pathology, whereas logopenic PPA is associated with AD pathology (intracellular tau tangles and extracellular amyloid protein plaques). Initially, symptomatic treatment was not started because his underlying etiology was unclear.
Outcome of the case. At his 1-year follow-up, the patient’s MMSE score was unchanged at 29/30 with normal memory. Although office cognitive testing was stable, the patient felt his language impairments were declining so empiric symptomatic treatment was discussed. Medications used for cognitive impairment included cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists. Our patient was started, off label, on the NMDA receptor antagonist memantine hydrochloride (HCl) (Namenda). Patients with AD have low acetylcholine levels as opposed to normal acetylcholine levels in PPA associated with tau or TDP-42 pathology.
Over time, the patient’s symptoms progressed and his language deteriorated further. His MMSE score declined to 23/30 with impaired memory.
In order to best guide symptomatic therapy, the need to identify the etiology of his symptoms was important. By this time, the florbetapir PET scan was FDA-approved for the evaluation of amyloid in patients with cognitive impairments. Note: This test was not covered by the patient’s insurance, but he had the financial means to pay for the test.
The florbetapir PET scan, completed 3 years after his initial symptoms, was positive. A positive scan is supportive of amyloid pathology. With his history and workup, the positive scan suggested a diagnosis of atypical AD (logopenic PPA). Based on the positive scan results, the patient’s care plan included the initiation of a cholinesterase inhibitor in addition to memantine HCl. Now, with evidence of amyloid pathology, the patient currently has the option of continuing with symptomatic treatment and pursuing a clinical trial focused on AD pathology.
Case Study #2: Patient With Short-Term Memory Loss
A 53-year-old female consulted the clinic for a history of gradual short-term memory loss of about 4 years duration. The symptoms became apparent when the patient was having difficulty with multitasking. Her work performance was affected because of decreased efficiency. At the onset of her cognitive symptoms, she was also experiencing anxiety and was started on an antidepressant by her primary care provider.
History. She has a history of a motor vehicle crash with head trauma and loss of consciousness 25 years prior to the onset of symptoms. She also has a medical history of diabetes diagnosed 4 years prior, hypertension, hyperlipidemia, obstructive sleep apnea, coronary artery disease, congestive heart failure, and osteoarthritis with bilateral knee replacements. Her mother, maternal uncles, and aunts were all clinically diagnosed with Alzheimer’s dementia. Based on the patient’s medical history, her previous head trauma, and her family history, she appeared to have an increased risk of AD.
Laboratory tests. Her MMSE score at her initial consultation was 27/30 with mildly impaired short-term recall. Her brain MRI was normal and did not reveal significant vascular changes. Laboratory testing was unremarkable. Her medical comorbidities remained stable.
Neuropsychological evaluation was completed and did not reveal a primary amnestic disorder, despite her initial complaints.
Diagnosis. She was given a diagnosis of nonamnestic mild cognitive impairments (MCI). It was also discovered in testing that the patient has a chronic personality disorder, which had not been previously diagnosed or treated.
With a company-sponsored voucher, the patient was able to have the florbetapir PET scan. The scan was negative, not supportive of excess amyloid in the cortical strip. A negative PET scan does not support a diagnosis of Alzheimer’s pathology to explain her cognitive complaints. The negative scan provided a great amount of relief to the patient.
Outcome of the case. One year later, her repeat cognitive testing showed improvements in the previously impaired areas, and she no longer met the clinical criteria for MCI. The current suspected diagnosis is pseudodementia versus vascular cognitive impairment. Her plan of care is focused on psychiatric treatment. Treatment with cholinesterase inhibitors and/or memantine HCl was not indicated based on the results of the florbetapir PET scan.
Cost of Symptomatic Treatment
To investigate the cost of an oral symptomatic treatment over 1 year, we chose donepezil (Aricept), a commonly prescribed cholinesterase inhibitor FDA approved for mild, moderate, and severe AD, and pulled costs using a local zip code:
• For generic donepezil 5 mg tablets, the cost for a 90-day prescription is $591.00, and for brand-name Aricept, $1,116.42.
• For the 10 mg tablet, a 90-day prescription for generic donepezil is $659.00, and for brand-name Aricept, $ 1,585.00.
• For the 23 mg brand-name Aricept, a 90-day prescription is $1,554.00.
For 1 year, a 10 mg generic prescription would cost $ 2,636.00, and $6,340.00 for brand-name Aricept. The cost for a 5-year prescription for symptomatic treatment for suspicion of AD is $13,180.00 for generic, or $31,700.00 for brand-name Aricept. A negative amyloid PET would preclude the indication for this as well as other symptomatic AD therapies.13
In our clinic, we had a patient with a clinical diagnosis of Alzheimer’s dementia. His postmortem diagnosis was hippocampal sclerosis without any pathological signs of AD. Hippocampal sclerosis symptoms can mimic AD. The patient was treated with symptomatic treatment, Aricept, for 14 years. Using the estimates above, the cost of symptomatic treatment for the patient’s clinical diagnosis would be roughly $80,000.13
AD is the most common form of dementia and accounts for 60% to 80% of such cases.2 The prevalence of AD is expected to increase in the aging population, leading to a greater economic burden. With such a significant projected increase in the prevalence of AD and the staggering financial cost to treat and care for AD patients, there is an urgent need to find a cure and develop tools for prevention. Individuals with complaints of cognitive changes should undergo a neurological workup directed by their primary care provider, psychiatrist, or a consulting neurologist.
It is challenging to evaluate patients who have very mild cognitive symptoms. Alzheimer’s dementia has been essentially a diagnosis of exclusion. Today in clinical practice, a diagnosis of AD initiates the start of symptomatic treatment even without pathological confirmation. Success of symptomatic treatment varies and side effects can frequently occur. The amyloid imaging PET scan benefits patient diagnosis and helps direct management and decision-making. In our experience, we have found that the patients with negative scans have benefited by avoiding unnecessary cost of medications and possible side effects. They also feel relief and are provided reassurance that their cognitive symptoms are not on the basis of AD pathology. A negative scan also triggers continued workup to find another cause for the cognitive symptoms.
References:
1. Cleveland Clinic. Alzheimer’s disease. www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/neurology/alzheimers-disease. Accessed November 2014.
2. Galvin, JE, Sadowsky CH. Practical Guidelines for the recognition and diagnosis of dementia. J Am Board Fam Med. 2012;25(3):367-382.
3. Sperling R. Can we detect Alzheimer’s disease 10 years before dementia and why would we want to? Alzheimers Dement. 2011;7(4):S805.
4. Alzheimer's Association. Alzheimer’s disease facts and figures. www.alz.org/alzheimers_disease_facts_and_figures.asp#cost. Accessed November 2014.
5. Administration for Community Living. Aging statistics. www.aoa.gov/Aging_Statistics/. Accessed November 2014.
6. Wollman DE, Prohovnik I. Sensitivity and specificity of neuroimaging for the diagnosis of Alzheimer’s disease. Dialogues Clin Neurosci. 2003;5(1):89-99.
7. Alzheimer’s Association. New diagnostic criteria and guidelines for Alzheimer’s disease. www.alz.org/research/diagnostic_criteria. Accessed November 2014.
8. Mattsson N, Andreasson U, Persson S, et al. CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement. 2013;9(3):251-261.
9. Formichi P, Battisti C, Radi E, et al. Cerebrospinal Fluid tau, A beta, and phosphyorylated tau protein for the diagnosis of Alzheimer’s Disease. J Cell Physiology. 2006;208(1):39-46.
10. Florbetapir [package insert]. Indianapolis, IN: Eli Lilly & Co; 2013.
11. Klunk W, Mathis C. The future of amyloid-beta imaging: a tale of radionuclides and tracer proliferation. Curr Opin Neurol. 2008;21(6):683-687.
12. Centers for Medicare and Medicaid Services. Proposed decision and memo for beta amyloid positron emission tomorgraphy in dementia and neurodegenerative disease (CAG-00431N). www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=265. Accessed January 2015.
13. Aricept (donepezil), Walgreens in zip code 32803. www.goodrx.com. Accessed January 2015.


