Atherosclerotic Peripheral Arterial Disease of the Lower Extremities: What Every Primary Care Physician Should Know
ABSTRACT: Peripheral arterial disease is a common circulatory condition in the United States that increases in prevalence with advancing age. Although the condition is well recognized, studies indicate that it is underdiagnosed, including in primary care practices. With the population of older adults continuing to increase, and many seeking care in such practices, it is essential for healthcare providers serving these patients to have increased awareness of the condition, be able to identify at-risk patients, understand strategies for ensuring an accurate diagnosis, and know the available treatment options, all of which are covered in this article in a question and answer format.
_____________________________________________________________________________________________________________________________________________
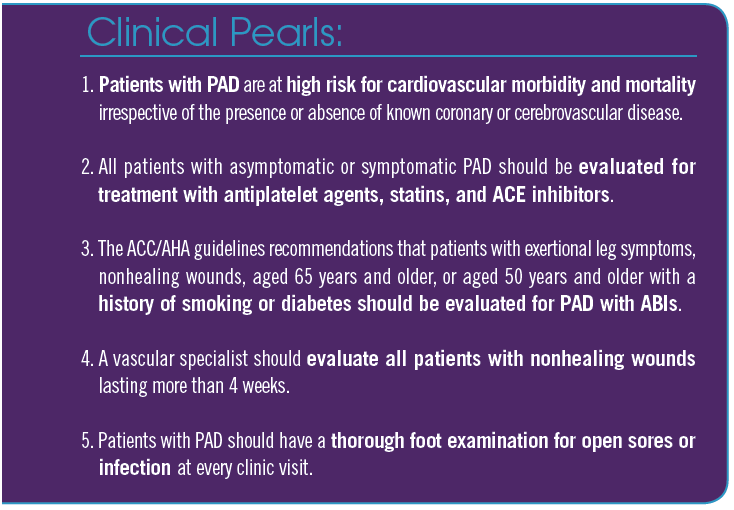
Peripheral arterial disease (PAD) is a condition characterized by atherosclerotic narrowing or occlusion of the arteries, reducing blood flow to the limbs, most commonly the lower extremities. In the United States, an estimated 8 to 12 million people have PAD.1 The condition is more common among those aged 50 years and older, with approximately 1 in 20 Americans in this age group affected.1 Therefore, it is not uncommon to encounter PAD in primary care clinics, though it likely remains underdiagnosed and undertreated.
In 2001, the PARTNERS (PAD Awareness, Risk, and Treatment: New Resources for Survival) program uncovered some eye-opening facts regarding PAD prevalence in primary care clinics throughout the United States.2 In this study, 6979 patients aged 70 years and older and those aged 50 to 69 years with a history of cigarette smoking or diabetes were screened for PAD using ankle brachial index (ABI) measures. An ABI of 0.90 or less was considered diagnostic of PAD. The study investigators discovered that 29% of all study participants had PAD, and 44% of participants only received the diagnosis after enrollment in the study, indicating a high prevalence of underdiagnosis. In addition, although 83% of patients with a prior PAD diagnosis were aware of their diagnosis, only 49% of their primary care physicians knew about it, despite documentation in the medical records. More recently, in 2009, PIPS (Peripheral Arterial Disease in Interventional Patients Study) investigators reported that PAD underdiagnosis persisted even in patients who were receiving specialty care for coronary artery disease (CAD), with a 15% prevalence of previously unrecognized PAD in this population.3 PAD was more likely to be overlooked in women, in those older than 70 years, and in those with other traditional cardiovascular risk factors.
Primary care physicians are on the frontlines of patient care, putting them in an ideal position to identify high-risk patients and diagnose PAD. To achieve this, a comprehensive understanding of this condition is essential. This article answers 10 key questions regarding PAD in primary care practices, examining persons at risk, modalities used for diagnosis, factors associated with morbidity and mortality, and treatment options.
1. Who should be tested for PAD?
Classically, we have been taught in medical schools to evaluate for PAD when patients report pain in their leg muscles upon walking, a condition known as intermittent claudication; however, the presence of this condition and the absence of pedal pulses are not sufficient findings to identify the wider spectrum of PAD. In the PARTNER study, PAD would have been missed in 85% of patients had physicians relied solely on the presence of intermittent claudication, as only 11% of patients with PAD presented with this condition.2 In addition, patients with intermittent claudication infrequently report this symptom, as they tend to attribute it to the normal process of aging.4 It has also been shown that a thorough clinical examination assessing for bruits (iliac, femoral, and popliteal) and a pulse examination (dorsalis pedis and posterior tibial artery) is not sufficient to include or exclude a PAD diagnosis.5 Based on such reports, multiple vascular societies, including the American College of Cardiology (ACC), the American Heart Association (AHA), the Society for Vascular Medicine, and the Society for Vascular Surgery, recommend that the following patient groups should get ABI testing to diagnose PAD: patients with exertional leg symptoms, patients with nonhealing wounds, anyone aged 65 years and older, and individuals aged 50 years and older with a history of smoking or diabetes.6
2. How can PAD present in your office?
PAD can present in many ways, ranging from an incidental finding of significant lower extremity PAD without any associated symptoms, to the well-recognized symptom of intermittent claudication, to the more severe findings of tissue loss or significant leg pain at rest. Many patients with significant PAD do not recognize their symptoms, as they have associated them with normal aging,4 and have subconsciously adapted to their chronic leg discomfort by decreasing their walking distance, speed, or both.7 The classic presentation of intermittent claudication, which accounts for only 10% to 30% of PAD cases seen in primary care clinics,2,7,8 typically presents as fatigue, aching, or burning pain in the calves, thighs, or buttocks that is brought on and exacerbated by walking or exercise and is relieved by rest.
Another not so uncommon PAD presentation is critical limb ischemia, which consists of pain at rest and/or the presence of nonhealing wounds (Figure). Pain at rest indicates significant compromise of blood circulation to the lower extremities, even while the patient is resting. This agonizing pain invariably affects the distal foot, forcing patients to spend their nights in a sitting position or dangling the affected leg off the side of the bed in a dependent position to enable gravity to maximize arterial blood flow. In many cases, a nonhealing or slow-healing ulcer that has been present for more than 4 weeks on patients’ feet or toes may be the first indication of significant underlying PAD. The inciting event of such an ulcer is usually a minor trauma that went unnoticed. In rare cases, PAD may remain unrecognized until an acute thrombosis of a significantly narrowed major lower extremity artery manifests the classic symptoms of acute ischemia. Such acute limb ischemia manifests as the five “Ps,” which is comprised of pain, pallor, pulselessness, paresthesia, and paralysis.

Figure. A nonhealing wound of the right great toe with dependent rubor (purplish/reddish discoloration of toes on dependent position), which is seen in critical limb ischemia.
3. What tests should you order when you suspect PAD, and when should they be undertaken?
As previously noted, only a small fraction of all patients with PAD have the classic symptoms of intermittent claudication, pain at rest, or tissue loss; thus, PAD may go unrecognized in many patients, increasing the risk of morbidity and mortality. To reduce these risks, screening for PAD is essential to identify these high-risk patients. Use of ABIs has been shown to be a highly superior screening tool compared with reliance on the presence of intermittent claudication to diagnose PAD. An ABI of less than 0.9 is widely used as an indicator of PAD, whereas an ABI of more than 1.3 is also considered abnormal and indicative of noncompressible diseased arteries that are usually calcified. All patients with intermittent claudication, leg pain at rest, or nonhealing lower extremity wounds for more than 4 weeks should be evaluated for PAD starting with a pulse examination and ABI with or without pulse volume recordings (PVRs) and/or toe brachial index (TBI) measures. Patients with risk factors for atherosclerotic disease and who present with atypical leg symptoms may have underlying PAD and would benefit from screening using ABIs as well.
4. Why is it important to diagnose PAD?
The presence of PAD significantly increases the risk of morbidity and mortality, including all-cause and cardiovascular-related mortality.9 In study by Criqui et al, patients with PAD had a 3-fold greater 10-year risk of all-cause death (relative risk [RR], 3.1; 95% confidence interval [CI], 1.9-4.9) and a 6-fold greater risk of cardiovascular-related death compared with patients without PAD (RR, 6.6; 95% CI, 2.9-14.9).10 In the REACH (Reduction of Atherothrombosis for Continued Health) registry, by 1 year, 21% of patients with PAD had developed a myocardial infarction (MI) or stroke, underwent hospitalization, or had a cardiovascular-related death, compared with 15% of patients with CAD.11 In another study, of all the patients undergoing vascular surgery at the Cleveland Clinic between 1978 and 1981 who were assessed for concomitant cardiovascular disease (CVD) with coronary angiograms, only 10% had normal coronary arteries and 28% had severe 3-vessel CAD requiring surgical intervention.12
Even the presence of isolated PAD without known CVD is associated with an increased risk of MI, ischemic stroke, and death.9,13,14 The high prevalence of PAD among primary care patients, as shown in the PARTNERS study,2 coupled with physician unawareness of this disease process leads to underdiagnosis. Moreover, these patients tend to receive less intense lipid-lowering treatments and fewer antihypertensive and antiplatelet medications when compared with those with known CVD; thus, these patients could tremendously benefit by aggressive risk-factor modification at the primary care level.
5. What should physicians do for their patients with PAD?
Once PAD is diagnosed, it is imperative that patients are made aware of their diagnosis and the prognosis it portends. Adequate time spent explaining the systemic nature of the disease process and its cardiovascular implications will increase the likelihood of patients cooperating with and participating in their own treatment planning. A systematic treatment strategy that incorporates the lifestyle modifications of smoking cessation and exercise with aggressive therapeutic control of hyperlipidemia, hypertension, and diabetes is most prudent. This requires a thorough initial physical examination that includes bilateral upper arm blood pressure measurements, cardiac auscultation, and peripheral pulse checks of the bilateral carotid, brachial, radial, ulnar, femoral, popliteal, posterior tibial, and dorsalis pedis arteries. Attention must be given to assessing both lower extremities for subtle signs of ischemia, such as dependent rubor, hair loss, toenail hypertrophy, and evidence of any tissue loss (ulcer or gangrene), during every clinic visit. Absence of a palpable lower extremity pulse should lead to an examination with a handheld continuous wave Doppler machine. Ordering appropriate blood work to check serum chemistries, including the patient’s lipid panel and hemoglobin A1c (HbA1c) levels, should be considered.
Multiple societies have developed and published treatment guidelines and therapeutic principles for patients with PAD.6,15 Almost all of these recommendations can be broadly divided into 2 categories. The first involves treating all identified and modifiable atherosclerotic risk factors to prevent or minimize cardiovascular morbidity and mortality (see question 6). The second involves treating patients’ PAD based on disease severity and symptoms using combinations of a supervised exercise program, various pharmacologic interventions, and revascularization procedures, as appropriate (see question 9).
6. What are the atherosclerotic risk factors and how can they be modified?
There are numerous atherosclerotic risk factors, including smoking, dyslipidemia, hypertension, and diabetes mellitus. What follows is a brief overview of how these risk factors can be modified.
• Smoking cessation. Smoking cessation is associated with a 36% reduction in the risk of mortality from cardiovascular events in patients with known atherosclerotic CAD.16 When smoking cessation is attempted with physician encouragement, treatment and regular follow-up is about 50 times more likely to be successful than without any physician intervention.17 A strong physician message with group sessions directed at behavior modification and either a single or combined drug therapy with nicotine replacement, bupropion, or varenicline is usually highly effective.18-22
• Dyslipidemia. In patients with PAD, statin use has been shown to result in a 25% risk reduction in cardiovascular events at 5 years, independent of their baseline low-density lipoprotein (LDL) cholesterol levels.23 Aggressive treatment of hyperlipidemia is also associated with a reduction in new or worsening claudication and improves pain-free walking distance in patients with PAD and intermittent claudication.24,25 The ACC/AHA guidelines recommend high-intensity statins for all patients with PAD who are younger than 75 years, and at least moderate-intensity statins for patients with PAD who are aged 75 years and older.26 This is recommended regardless of patients’ baseline LDL cholesterol levels. In addition to reducing the risk of cardiovascular morbidity and mortality, statins have also been shown to reduce amputation rates in patients with PAD.26
• Hypertension. Controlling the blood pressure of patients with PAD using any pharmacological means is beneficial, as it slows atherosclerotic disease progression and lowers the risk of cardiovascular morbidity. According to the HOPE (Heart Outcome Prevention Evaluation) study, angiotensin-converting enzyme (ACE) inhibitors are particularly effective.27 In the study, ACE inhibitors led to a 22% risk reduction in MI, stroke, or vascular death, as compared with placebo.
• Diabetes mellitus. Uncontrolled diabetes is associated with a rapid progression of PAD and an increased risk of amputation and mortality.28,29 With every 1% increase in HbA1c level, the risk of PAD increases by a staggering 28%.30 Not surprisingly, better control of diabetes translates to slowed disease progression and fewer complications in patients with PAD. In UKPDS (UK Prospective Diabetes Study), lowering blood glucose levels to a goal median HbA1c of 7.0% using intensive therapy, compared with a median HbA1c of 7.9% using conventional therapy, decreased the overall microvascular complication rate by 25%.31 The American Diabetes Association recommendations, which are also endorsed by other PAD treatment guidelines, are to maintain an HbA1c level of less than 7.0% to reduce microvascular events and complications in patients with PAD.32
7. How do you monitor PAD?
A thorough history and physical examination with questions directed towards symptoms of intermittent claudication, any change in walking distance before the onset of pain, skin discolorations, healing problems, or worsening of any previous lower extremity ulcers, may indicate improvement, stability, or deterioration of preexisting PAD. The most cost-effective and noninvasive modality to monitor PAD is measuring ABIs. A change in ABI of more than 0.15 is considered significant and should warrant further investigation when accompanied by worsening symptoms. In patients with heavily calcified vessels, TBIs can serve the same purpose.
8. When should a patient with PAD be referred to a vascular specialist?
Patients who may benefit from a consultation with a vascular specialist include those with severe PAD at presentation with pain at rest or tissue loss in the form of a nonhealing ulcer; those with worsening and severe lifestyle-
limiting claudication; and those with disease progression even while on pharmacological treatments to modify their risk factors. A vascular specialist will be able to determine whether such patients might benefit from some form of revascularization for their PAD. An asymptomatic patient who has had a revascularization procedure and is noted to have a decrease in his or her ABIs on a follow-up visit may also benefit from a referral to a vascular specialist for further investigation and treatment, if necessary.
9. What treatment options are available for PAD?
In addition to the aforementioned treatments for risk factor modification (see question 6), a patient with PAD should be treated with various combinations of a supervised exercise program, pharmacological treatments, and revascularization with either endovascular or open surgery, if needed. What follows is a review of these treatment options.
Supervised Exercise Program
Supervised exercise therapy is more effective than an unsupervised exercise program.33 In a large meta-analysis, a supervised walking exercise program consisting of sessions lasting at least 30 minutes each, undertaken 3 times weekly for at least 6 months, improved participants’ walking distance to onset of claudication pain by 179%, and the distance until experiencing maximal claudication pain increased by 122%.34 Similar walking exercise programs should be part of every patient’s treatment, provided he or she can walk. Supervised exercise therapy has been associated with improvement in quality of life even in
asymptomatic patients.
Pharmacological Treatment
Numerous pharmacologic treatments can be considered, including antiplatelet therapy, cilostazol, pentoxifylline, and ACE inhibitors.
• Antiplatelet therapy. The main benefits of antiplatelet therapy in patients with PAD are mostly due to its secondary prevention of MI and strokes, as supported by the 23% risk reduction of nonfatal MI, nonfatal stroke, and vascular death observed by the Antithrombotic Trialists’ Collaboration overview of 287 studies involving 135,000 patients.35 In the CAPRIE (Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events) trial, clopidogrel was found to have a modest advantage over aspirin in reducing the incidence of stroke, MI, or vascular death in patients with symptomatic PAD or other atherosclerotic vascular disease with recent MI or stroke.36 All the current PAD guidelines strongly recommend prescribing antiplatelet therapy for patients with PAD. The ACC/AHA guidelines strongly recommend antiplatelet therapy to reduce the risk of MI, stroke, and vascular death in individuals with symptomatic lower extremity PAD, including those with intermittent claudication or critical limb ischemia, prior lower extremity revascularization (endovascular or surgical), or prior amputation for lower extremity ischemia (class IA).6
• Cilostazol. Cilostazol is an FDA-
approved medication for the management of intermittent claudication. This phosphodiesterase inhibitor, which also acts like a vasodilator, works by inhibiting platelet aggregation, thrombin formation, and smooth muscle proliferation. A dose of 100 mg twice daily has been shown to improve maximal walking distance by 50.7%, as compared with 24.3% with placebo.37 This drug is recommended as a first-line treatment for the pharmacological management of intermittent claudication; however, caution should be taken not to use this agent in patients with heart failure, as it has been associated with increased death in this subgroup of patients.4,6
• Pentoxifylline. Pentoxifylline is a methylxanthine derivative that decreases blood viscosity by improving erythrocyte deformability. It also has anti-inflammatory and antiproliferative effects. Although recommended as a second-line treatment for intermittent claudication by the ACC/AHA guidelines,6 its clinical efficacy remains inconsistent (similar to placebo) in various studies.38
• ACE inhibitors. In a 2013 randomized controlled trial, the ACE inhibitor ramipril showed improvement in peak walking time in patients with intermittent claudication, as compared with placebo alone.39 This finding indicates its benefits for patients with PAD extend beyond its control of hypertension.
Revascularization
As previously noted, revascularization should be reserved for individuals with acute limb ischemia, critical limb ischemia (pain at rest and/or tissue loss), and those with severe lifestyle-limiting claudication despite best medical management. Once the patient is medically optimized for an intervention necessitated by one of the aforementioned criteria, the best modality for revascularization must be individualized. This is often guided by the location and length of the lesion affecting the vasculature, as well as the physiological and functional status of the patient.
• Endovascular options. Generally, endovascular therapy is considered the preferred modality if the patient’s anatomy is favorable (ie, there is short-segment focal disease that does not involve regions of repetitive movement, such as the groin and knees). In such cases, the outcomes are equivalent to those achieved with open surgical procedures.4 However, endovascular therapy is less invasive, has fewer complications, and often has shorter hospital stays than open surgical procedures. With the advent of covered and drug-eluting stents, the durability of endovascular therapy has significantly improved.
• Open surgical endarterectomy or bypass. Long-segment lesions and those involving the arterial segments in the groin (ie, common femoral artery) and behind the knee (ie, popliteal artery) are best served using open surgical reconstructions using a bypass or an endarterectomy and patch repair. Open surgeries are more invasive and have a higher complication rate, but they may be more durable than endovascular options in certain situations, such as when there is long-segment arterial occlusion or the common femoral artery segment is involved.
10. What is on the horizon in the care for PAD patients?
There are 2 areas in PAD care where there are evolving developments that primary care physicians should be familiar with: angiogenesis and compression therapy.
• Angiogenesis. Angiogenesis is defined as the physiological process through which new blood vessels form from the preexisting vasculature. There has been considerable growth in this field over the past 3 decades due to its potential to combat a variety of diseases, including PAD.40 Two modalities of stimulating angiogenesis in patients with critical limb ischemia when no revascularization options are available are under investigation. The RESTORE-CLI trial, a randomized, double-blind multicenter phase II trial, compared the effectiveness of injecting aspirated bone marrow mononuclear cells into 20 different sites in the ischemic lower extremities versus sham aspiration and no injection.41 Injection of the mononuclear cells to stimulate angiogenesis was associated with an increased time to treatment failure and amputation-free survival. There was also a decreased incidence of major amputation in the treated group at 19% versus 43% for controls.41 Therapeutic angiogenesis can also be achieved by injecting angiogenic growth factors as recombinant protein or naked plasmid DNA to increase the vascular endothelial growth factor concentration in the ischemic limb, thereby improving endothelial function, blood flow reserve, and collateral vessel development.42,43
• Compression therapy. Treatment of stable claudication by applying intermittent mechanical compression to the calf muscle using a new portable mechanical compression device has been studied in a randomized trial.44 In the study, patients received intermittent mechanical compression in addition to medical therapy or medical therapy alone. After 1 month, those receiving concomitant compression therapy experienced significant improvements in initial claudication distance, absolute claudication distance, and postexercise ABIs.44 Based on their findings, the investigators conclude that intermittent mechanical compression may be a useful approach for patients with persistent claudication despite standard medical treatment.
Conclusion
PAD can be difficult to diagnose, particularly in asymptomatic patients. Primary care physicians may not think to check for PAD if a patient does not present with the hallmark symptom of intermittent claudication or demonstrate tissue loss or severe leg pain at rest; however, any at-risk patients or those with findings suspicious of PAD should be carefully evaluated for this condition. Timely screening ensures early diagnosis and treatment, reducing the risks of morbidity and mortality while improving patients’ quality of life.
References:
1. US Department of Health and Human Services. Facts about peripheral arterial disease (P.A.D.). www.nhlbi.nih.gov/health/public/heart/pad/docs/pad_extfctsht_general_508.pdf. Accessed June 19, 2014.
2. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317-1324.
3. Moussa ID, Jaff MR, Mehran R, et al. Prevalence and prediction of previously unrecognized peripheral arterial disease in patients with coronary artery disease: the Peripheral Arterial Disease in Interventional Patients Study. Catheter Cardiovasc Interv. 2009;73(6):719-724.
4. Norgren L, Hiatt WR, Dormandy JA, et al.
Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1-S75.
5. Khan NA, Rahim SA, Anand SS, Simel DL,
Panju A. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295(5):536-546.
6. Rooke TW, Hirsch AT, Misra S, et al; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society for Vascular Medicine; Society for Vascular Surgery. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58(19):2020-2045.
7. McDermott MM, Greenland P, Liu K, et al.
Leg symptoms in peripheral arterial disease:
associated clinical characteristics and functional impairment. JAMA. 2001;286(13):1599-1606.
8. McDermott MM. The magnitude of the problem of peripheral arterial disease: epidemiology and clinical significance. Cleve Clin J Med. 2006;73(Suppl 4):S2-S7.
9. Golomb BA, Dang TT, Criqui MH. Peripheral
arterial disease: morbidity and mortality
implications. Circulation. 2006;114(7):688-699.
10. Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381-386.
11. Steg PG, Bhatt DL, Wilson PW, et al; REACH Registry Investigators. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297(11):1197-1206.
12. Hertzer NR, Beven EG, Young JR, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984;199(2):223-233.
13. McDermott MM, Feinglass J, Slavensky R, Pearce WH. The ankle-brachial index as a predictor of survival in patients with peripheral vascular disease. J Gen Intern Med. 1994;
9(8):445-449.
14. McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87(2-3):119-128.
15. Norgren L, Hiatt WR, Dormandy JA, et al;
TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26(2):81-157.
16. Critchley J, Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev. 2003;(4):CD003041.
17. Law M, Tang JL. An analysis of the effectiveness of interventions intended to help people stop smoking. Arch Intern Med. 1995;155(18):1933-1941.
18. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE; Lung Health Study Research Group. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233-239.
19. Tonstad S, Farsang C, Klaene G, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J. 2003;24(10):946-955.
20. Jorenby DE, Leischow SJ, Nides MA, et al.
A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340(9):685-691.
21. Jorenby DE, Hays JT, Rigotti NA, et al; Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56-63.
22. Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121(2):221-229.
23. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22.
24. Pedersen TR, Kjekshus J, Pyörälä K, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S). Am J Cardiol. 1998;81(3):333-335.
25. Mohler ER 3rd, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108(12):1481-1486.
26. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic
Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. Accessed June 19, 2014.
27. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145-153.
28. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287(19):2570-2581.
29. Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24(8):1433-1437.
30. Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421-431.
31. Turner RC, Holman RR, Cull CA, et al; UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853.
32. American Diabetes Association. Peripheral arterial disease (PAD). www.diabetes.org/living-with-diabetes/complications/heart-disease/
peripheral-arterial-disease.html. Edited April 30, 2014. Accessed June 19, 2014.
33. Bendermacher BL, Willigendael EM, Teijink JA, Prins MH. Supervised exercise therapy versus non-supervised exercise therapy for
intermittent claudication. Cochrane Database Syst Rev. 2006;(2):CD005263.
34. Gardner AW, Poehlman ET. Exercise
rehabilitation programs for the treatment
of claudication pain. A meta-analysis.
JAMA. 1995;274(12):975-980.
35. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71-86.
36. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;
348(9038):1329-1339.
37. Pande RL, Hiatt WR, Zhang P, Hittel N, Creager MA. A pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermittent claudication. Vasc Med. 2010;15(3):181-188.
38. Dawson DL, Cutler BS, Hiatt WR, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000;109(7):523-530.
39. Ahimastos AA, Walker PJ, Askew C, et al.
Effect of ramipril on walking times and
quality of life among patients with peripheral artery disease and intermittent claudication:
a randomized controlled trial. JAMA. 2013;
309(5):453-460.
40. Adair TH, Montani JP. Angiogenesis. www.ncbi.nlm.nih.gov/books/NBK53242.
Accessed June 19, 2014.
41. Powell RJ, Marston WA, Berceli SA, et al. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trial.
Mol Ther. 2012;20(6):1280-1286.
42. Isner JM, Pieczek A, Schainfeld R, et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996;348(9024):370-374.
43. Baumgartner I, Pieczek A, Manor O, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97(12):1114-1123.
44. de Haro J, Acin F, Florez A, Bleda S, Fernandez JL. A prospective randomized controlled study with intermittent mechanical compression of the calf in patients with claudication.
J Vasc Surg. 2010;51(4):857-862.
Aditya Sharma, MD, is assistant professor of medicine and
a vascular medicine physician, Division of Cardiovascular Medicine, University of Virginia, Charlottesville, VA.
Amit Jain, MD, is a vascular surgery fellow, Department of Surgery, University of Virginia, Charlottesville, VA.
Kanwar Singh, MD, is associate professor of medicine and a peripheral vascular and interventional cardiologist, Division of Cardiovascular Medicine, University of Virginia, Charlottesville, VA.
John Waites, MD, is a vascular medicine physician, Wheeler Heart and Vascular Center, Springfield, MO.


