Peer Reviewed
The Role of Probiotics in Improving Gastrointestinal Health
AFFILIATIONS:
1Department of Medicine, Research and Innovation, War Memorial Hospital, Sault Sainte Marie, Michigan
2Intensive Care, War Memorial Hospital, War Memorial Hospital, Sault Sainte Marie, Michigan
CITATION:
Samuel B, Mazzuca C, Gakstatter C. The beneficial role of probiotics in improving gastrointestinal health. Consultant. 2021;61(11):e1-e7. doi:10.25270/con.2021.04.00001
Received June 6, 2016. Accepted August 23, 2016. Published online April 7, 2021.
DISCLOSURES:
The authors report no relevant financial relationships.
CORRESPONDENCE:
Bensson Samuel, MD, PhD, DBA, CHCQM, 500 Osborn Boulevard, Sault Ste. Marie, MI 49783 (bsamuel@wmhos.org)
The gastrointestinal (GI) tract is home to microorganisms that exist in close relation with host cells. The microbes in the gut are estimated to comprise 1000 to 1200 bacterial species and at least 100 trillion bacteria, the majority of which are in the colon.1 The varied functions carried out by these bacteria are the synthesis of vitamins, the fermentation of dietary carbohydrates, the metabolism of bile and host hormones, and development of the immune system in the GI tract.2,3 These bacteria also play a key role in stress response.
Bidirectional brain-gut interaction mechanisms are well documented in the literature; however, the interactions of microbes and pathogenic organisms have only been recognized within the past few years, and theories continue to expand.2 The brain-gut axis has been described as an imaginary line between the brain and the gut, and it is one of the new frontiers of neuroscience.4
Gut homeostasis can be directly influenced by intestinal microbiota through the regulation of bowel motility and modulation of intestinal pain, immune responses, and nutrient processing.5,6 The central nervous system (CNS) additionally works by controlling various gut functions including motility, secretion, blood flow, and gut-associated immune function in response to psychological and physical stressors. Communication from intestinal microbiota to the host can occur through multiple mechanisms, including the use of epithelial cells, receptor-mediated signaling, and increasing intestinal permeability through direct stimulation of host cells in the lamina propria.
Microbiota in the gut is often referred to as the “second genome” or the “second brain” and could influence mood in ways that scientists are just now beginning to understand. Unlike with inherited genes, it may be possible to reshape or cultivate this second genome.4
This article examines evidence-based theories about the gut microbiota’s involvement in the brain-gut axis and discusses its effect on human health, especially psychological components such as stress, anxiety, and depression.
The Role of Probiotics
What is the role of microbiota in building immunity and maintaining homeostasis during metabolic and psychological stress?
Beginning from birth, the microbiota in the GI tract begins developing gut-associated immunity and immune functions. Interactions between the microbiota and mucous membranes associated with the immune system are facilitated through adaptive and natural immune response.3 In humans, the lower intestine contains 100 trillion to 1 quadrillion bacteria; that is, there are 10 to 100 times more bacteria in the gut than there are somatic cells in the human body.7
Changes in the biodiversity of the gut microbiota have been associated with consequences on host cell health and development.8 The composition of a bacterial host is determined by both environmental and genetic factors.3 Changes in the environmental composition can upset the balance between beneficial symbiosis and detrimental pathobionts, which leads to inflammation.9
Today, humans have decreased contact with natural biodiversity, which is negatively affecting commensal bacteria and reducing their immune modulation and immunoregulatory benefit.10 For example, our food sources are modified to be safe for consumers by the time the food reaches store shelves. People also depend on sources of water that have been filtered and purified. Before regulations, people were responsible for growing and hunting their own food, which they would process accordingly, increasing the chances of natural bacterial exposure. This allowed for the body to naturally build immunity to different bacterium types. The reduced contact of biodiversity leaves the immune system unexposed to various antigens, resulting in changes to the immune response and microbiota, further leading to stress and depression.10
During stress, there is a dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis.11 The HPA axis consists of an interaction between the hypothalamus, pituitary, and adrenal glands. The hypothalamus controls the autonomic nervous system and regulates the endocrine system, including the pituitary. The autonomic nervous system regulates bodily functions including rhythms, hunger and thirst, and temperature. The pituitary is responsible for hormone control. The adrenal cortex is responsible for releasing hormones such as cortisol, which functions to regulate blood pressure and stress response.12 Increased cortisol acts as an immunosuppressant and can make the body vulnerable to infection. During stressful times, the cortisol level in our body goes up, which could affect the gut permeability. Chronic psychological stress can cause elevation of cortisol levels, which can then cause intestinal barrier dysfunction and impair host defense mechanisms mediated by corticotrophin-releasing factor (CRF) and mast cells.13
By downregulating the response of stress hormones, microbiota can keep our body in an immunocompetent state. Studies have shown that mice with a decreased variety of commensal bacteria have an exaggerated response to stress, causing inflammation.14 The inhibition of stress secondary to infection would be inhibited and the immune response is modulated as the probiotics secrete defensins, enhance barrier function, reduce luminal pH and overall health (Figure 1). Treatment of the mice with probiotics decreased their stress hormone levels and prevented symptoms of depression from clinically presenting.14 There is evidence that the microbiota acts as a modulator of this immune-related increase in anxiety-like behavior. The same report stated that treatment with probiotics, including Bifidobacterium longum, helped alleviate the anxiety-like behavior, further raising the possibility that the use of probiotics reduces anxiety and inflammation.14
Figure 1. Interactions Between Genes and Their Environment in IBD

Source: Aldhous MC. Gene–environment interactions in inflammatory bowel disease: microbiota and genes. Frontline Gastroenterol. 2012;3(3):180-186. https://dx.doi.org/10.1136%2Fflgastro-2011-100097
Bidirectional Signaling
Metabolic byproducts of intestinal bacteria act at the local enteric nervous system and cause a change in the transit time through the gut.15,16 Depending on the species and the type of byproduct (small fatty acids), patients can experience diarrhea or constipation.17 Microorganisms can communicate with mammalian cells via interkingdom signaling using hormone-like compounds such as peptides and monoamines, including epidermal growth factor, insulin, and small diffusible signaling molecules called autoinducers.3 The homology of the microbial autoinducer 3–QseC signaling system with the mammalian noradrenergic signaling system causes QseC to be activated by norepinephrine, allowing for interkingdom signaling with particular relevance for brain-gut interactions during stress. Homology exists between the mammalian noradrenergic signaling system and the microbial autoinduce 3-Qsec signaling system, which allows norepinephrine to activate. This interkingdom signaling becomes apparent in the brain-gut interactions that occur during stress. Signals released by the host have receptors with intracellular activation systems, which suggests the microbes could be directly acted on by the host.3 It has been shown that mice with increased social stressors experience a downregulation in autoreceptors, which enhances serotonin release presynaptically but decreases the rate of reaching postsynaptic neurons.
Serotonin is a monoamine associated with the GI tract. An increase in serotonin in humans can lead to symptoms such as diarrhea, nausea, vomiting, headache, and a change in blood pressure. This change is further associated with GI disease, including irritable bowel syndrome (IBS), functional dyspepsia, and gastroesophageal reflux disease.
Interaction of Probiotics and the CNS
Multiple pathways of communication are used between the gut and the brain, including immune, humoral, and neural mechanisms, and alterations in bidirectional gut-brain interactions. These pathways are implicated in both functional GI disorders and CNS disorders such as autism, anxiety, and depression.18 Psychological or social stress can cause changes in the composition of microbiota in the enteric system (GI tract) that is induced by the CNS. The emotional motor system (CNS-mediated effects on the enteric system) comprises several parallel output systems that include the sympathetic and parasympathetic branches of the autonomic nervous system, the HPA axis, and endogenous pathways that modulate pain and discomfort, which mediate the effect of emotional states on a wide range of bodily systems, including GI tract function .12
The CNS plays a prominent role in regulating immune and bacterial function in the gut. This system of transmitted pulses directly effects luminal bacterial behavior through the release of various signaling molecules from epithelial cells into the gut lumen and indirectly impacting changes to the microbial environment through secretions and spontaneous movements.3,19 Various hormones such as cortisol and cytokines affect the epithelial cells, and mucosal cells interact with the GI biome and secrete and process short chain fatty acids that affect mood, cognition, and emotions (Figure 2). One study showed that the activation of the sympathetic system causes secretion of interleukin-6 (a pro-inflammatory protein) from immune cells. This increase leads to the activation of the HPA axis and suppression of the immune inflammatory reactions, leading to GI problems including colitis.12 Based on this information, there is evidence that stress-induced activation of any one of the parallel systems can result in changes in the microbial environment and composition, increasing the chances of GI disease.20
Figure 2. Pathways Involved in Bidirectional Communication Between the Gut Microbiota and the Brain
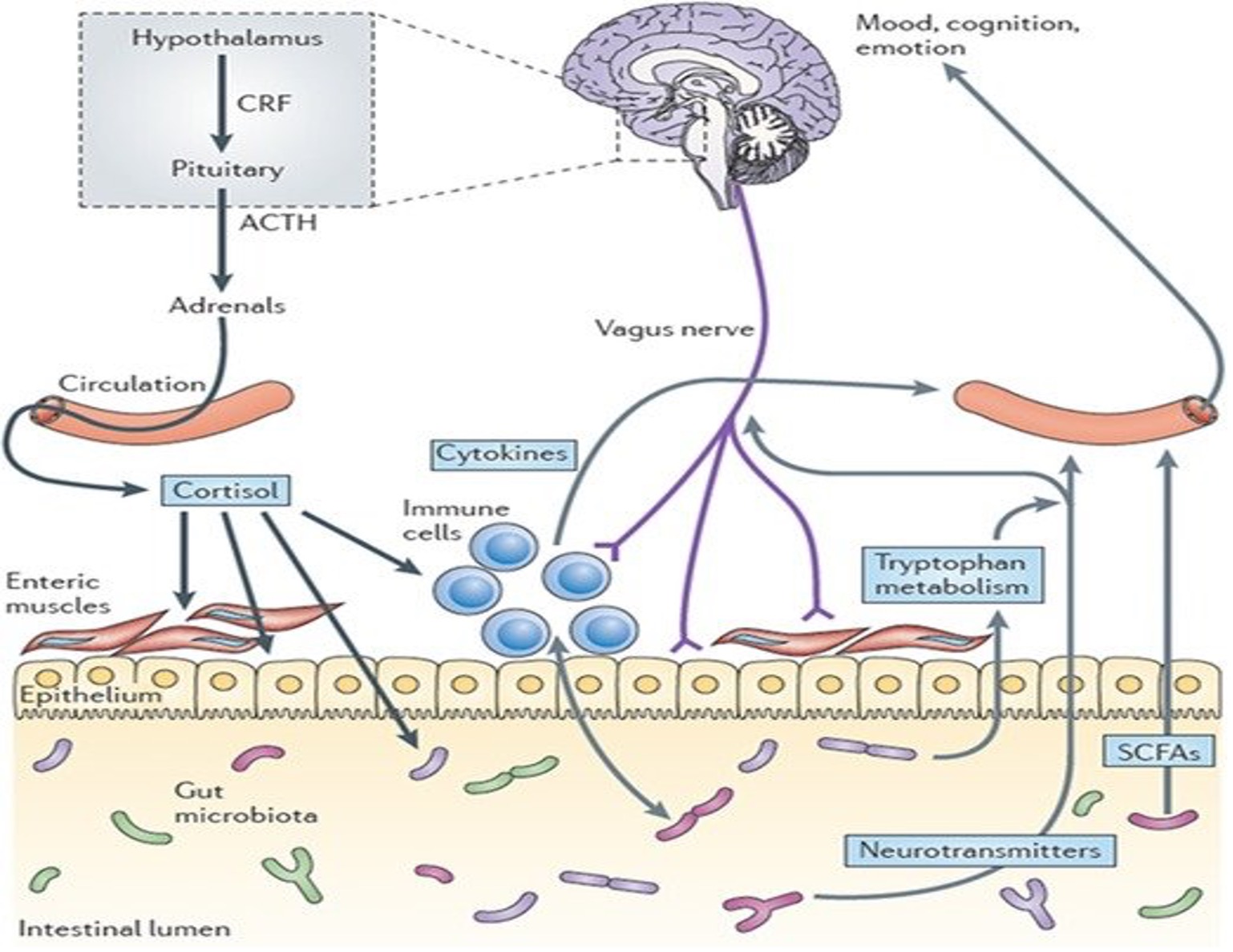
Source: Cryan JF, TG Dinan. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712. https://doi.org/10.1038/nrn3346
Improvement of Cerebral Function
Gut microbiota is vital to healthy brain development. The microbiota may influence areas of the brain involved in the human response to stress and control of stress-related conditions such as anxiety and depression.21 Microbial colonization during the perinatal period initiates signaling mechanisms that affect neuronal circuits involved in motor control and anxiety-like behavior. One study suggested that the differences in anxiety and motor behavior observed between germ-free mice and specific pathogen-free (SPF) mice could be attributable to the benefits of early colonization resulting in behavior modification.22 The mice were subjected to the light-dark box test and elevated plus maze test. It was hypothesized that early exposure to microbiota would result in different behavior. It was found that the germ-free mice that were not exposed to any microbiota that spent more time in the light end of the box. The germ-free mice also engaged in riskier behavior than the SPF mice. The data were determined based on the number of visits to the open arms of the plus maze experiment.22 Based on this study, it is evident that being exposed to microbiota early on influences behavior. The role of intestinal microbiota on the development of stress, emotion, and pain modulation systems has been identified through neuroplasticity changes seen within the emotion regulation regions and signaling systems of the brain.22 Studies have also shown that probiotic consumption may have a positive effect and can improve psychological symptoms of depression, anxiety, and stress.23
Communication of Enteric Microbiota to the Host
The enteric microbiota communicates with the host through multiple mechanisms, including epithelial-cell, receptor-mediated signaling and increased intestinal permeability from direct stimulation of host cells in the lamina propria.
Commensal bacteria play an important role in maintaining the integrity of the GI tract in situations of stress or disease. Whenever intestinal permeability is increased out of the control of commensal bacteria, and unknown antigens are allowed into the mucosa, an inflammatory reaction is destined to occur.21 Stress-induced changes in permeability involve activation of glial and mast cells of the gut, overproduction of interferon-γ, changes in the morphology of the colonic epithelium via reduced expression of tight junction protein 2 (zona occludens-2), and by occlusion of important components of the intestinal tight junction.15 The increased permeability allows for rearrangement of the bacteria outside the lumen of the enteric tract. Here, bacteria communicate by interaction through the local enteric nervous system (ENS), which is part of the autonomic nervous system that is housed in the gut and is responsible for gut motility and other normal gut functions. Toll-like receptor signaling, a type of signaling in the immune system, and signaling from bipeptides or tripeptides are used by luminal bacteria to influence the gut.24,25
Microbial signaling molecules could interact directly with afferent nerve terminals in situations where intestinal permeability is enhanced such as with inflammation or stress. Their signal could be relayed to neurons within the intestinal wall by transducer cells that respond to cells that respond to mechanical, thermal, photic, or chemical stimulus.3 One such cell is the enterochromaffin cell, which is scattered throughout the intestinal epithelium and acts as a bidirectional signal transducer secreting serotonin.26
Future Role in Human Health
Based on 2 randomized trials on the use of probiotics, there were improvements of symptoms in patients with inflammatory bowel disease (IBD) and IBS.27,28 Patients experienced a reduction in abdominal pain and discomfort and lower levels of pain-induced stress. In another study on individuals with chronic fatigue syndrome, administration of probiotics over a 2-month trial resulted in fewer anxiety-related symptoms.29
In the past few years, enormous strides have been made in understanding the role of microbiota in anxiety, IBD, brain functions, and metabolic disorders. One study showed that the administration of fermented milk product with probiotics produced a positive difference in brain regions responsible for emotion and sensation.30 The microbiota is likely part of the answer to a multifaceted approach to human health and will require more research. Several studies have been compared and analyzed to find that probiotics yield a positive effect on mood and stress.23,31 Given the current understanding, probiotics could potentially be incorporated into treatment plans for psychological disorders including anxiety and depression.23
Besides the ingestion of probiotics, numerous lifestyle modifications can be implemented to prevent dysbiosis (an increased ratio of harmful to beneficial bacteria). One of the most obvious but often overlooked modification is to diversify one’s diet with food high in prebiotics and dietary fiber, such as lentils, chickpeas, beans, bananas, kombucha, kefir, and kimchi.31 These foods have been associated with the production of short-chain fatty acids, the metabolism of which in the colon has been attributed to the concomitant reduction of the luminal pH, which by itself inhibits pathogenic microorganisms, increases the absorption of some nutrients, and plays an important role in the maintenance of the gut barrier.31 Another contributor to dysbiosis is the consumption of more than the recommended daily amount of alcohol. Of the choices of alcoholic drinks, moderate red wine consumption has been linked to a positive effect on the gut’s microbiota thanks to red wine’s abundance of polyphenols.32 Figure 3 is a good depiction of how the GI system can affect the body’s response to stress through the HPA pathway, endocrine secretion of hormones, and interaction with the nervous system.
Figure 3. Mechanisms and Pathways of Gut Dysbiosis and Altered Brain Function
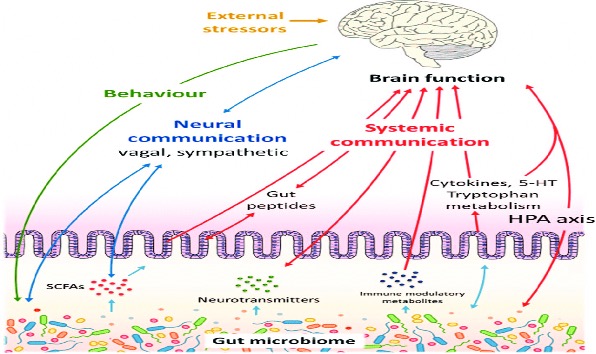
Source: Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21(6):738-748. https://doi.org/10.1038/mp.2016.50
Smoking has detrimental effects on nearly every organ in the body, including the gut, via its propensity to induce inflammatory reactions.33 It has been shown that smoking cessation induces a profound and robust shift in the intestinal microbial composition.34 High levels of stress also can induce harmful effects on the body. In the gut, chronic high stress levels can increase sensitivity, reduce perfusion and alter the gut microbiota.35 The probiotics diminish the immune response to infection and suppress the release of interleukins, which would otherwise be exaggerated (Figure 4). Studies in mice have shown that different types of stress, such as isolation, crowding, and heat stress, can reduce gut flora diversity and alter gut profiles by increasing potentially harmful bacteria such as Clostridioides difficile and reducing beneficial populations of bacteria such as Lactobacillus.36
Figure 4. Mechanisms Involved in Probiotic-Induced Protection Against Intestinal Dysbiosis

Source: Zhang M, Sun K, Wu Y, Yang Y, Tso P, Wu Z. Interactions between intestinal microbiota and host immune response in inflammatory bowel disease. Front Immunol. 2017;8:942. https://doi.org/10.3389/fimmu.2017.00942
Probiotic usage rests heavily upon the degree of anticipated benefit, the clarity of the available data showing a clear benefit, available alternatives, costs, and the preferences of the patient. As of now, no probiotic strategy is considered as either the standard of care or primary treatment for any of the medical conditions described herein. Enthusiasm for probiotics has outpaced the scientific evidence; therefore, large, well-designed, multicenter controlled clinical trials are in great need to further clarify the role of specific probiotics in different well-defined patient populations. In recent studies, the disease states that have shown the most benefit from the use of probiotics include pouchitis,37 ulcerative colitis,38 infectious diarrhea,39 prevention of C difficile infection,40 functional constipation,41 and IBS.28
References
1. Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe. 2009;15(6):285-289. https://doi.org/10.1016/j.anaerobe.2009.09.007
2. Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37(1):42-47. https://doi.org/10.1097/00004836-200307000-00012
3. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306-314. https://doi.org/10.1038/nrgastro.2009.35
4. Foster JA. Gut feelings: bacteria and the brain. Cerebrum. 2013;2013:9. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3788166/
5. Husebye E, Hellström PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol. 2001;280(3):G368-G380. https://doi.org/10.1152/ajpgi.2001.280.3.g368
6. Ait-Belgnaoui A, Han W, Lamine F, et al. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut. 2006;55(8):1090-1094. https://doi.org/10.1136/gut.2005.084194
7. Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61(3):355-361. https://doi.org/10.1038/sj.ejcn.1602546
8. Doré J, Simrén M, Buttle L, Guarner F. Hot topics in gut microbiota. United European Gastroenterol J. 2013;1(5):311-318. https://doi.org/10.1177/2050640613502477
9. Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology. 2010;139(6):1816-1819. https://doi.org/10.1053/j.gastro.2010.10.036
10. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25(3):397-407. https://doi.org/10.1016/j.bbi.2010.10.023
11. Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47(6):861-869. https://doi.org/10.1136/gut.47.6.861
12. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59-65. https://doi.org/10.1038/nature08821
13. Teitelbaum AA, Gareau MG, Jury J, Yang PC, Perdue MH. Chronic peripheral administration of corticotropin-releasing factor causes colonic barrier dysfunction similar to psychological stress. Am J Physiol Gastrointest Liver Physiol. 2008;295(3):G452-G459. https://doi.org/10.1152/ajpgi.90210.2008
14. Lyte M, Li W, Opitz N, Gaykema RPA, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89(3):350-357. https://doi.org/10.1016/j.physbeh.2006.06.019
15. Barbara G, Stanghellini V, Brandi G, et al. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100(11):2560-2568. https://doi.org/10.1111/j.1572-0241.2005.00230.x
16. Dass NB, John AK, Bassil AK, et al. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil. 2007;19(1):66-74. https://doi.org/10.1111/j.1365-2982.2006.00853.x
17. Ghoshal UC, Srivastava D. Irritable bowel syndrome and small intestinal bacterial overgrowth: meaningful association or unnecessary hype. World J Gastroenterol. 2014;20(10):2482-2491. https://doi.org/10.3748/wjg.v20.i10.2482
18. Appleton J. The gut-brain axis: influence of microbiota on mood and mental health. Integr Med (Encinitas). 2018;17(4):28-32. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc6469458/
19. Walters M, Sperandio V. Autoinducer 3 and epinephrine signaling in the kinetics of locus of enterocyte effacement gene expression in enterohemorrhagic Escherichia coli. Infect Immun. 2006;74(10):5445-5455. https://doi.org/10.1128/iai.00099-06
20. Baritaki S, de Bree E, Chatzaki E, Pothoulakis C. Chronic stress, inflammation, and colon cancer: a crh system-driven molecular crosstalk. J Clin Med. 2019;8(10):1669. https://doi.org/10.3390/jcm8101669
21. Neufeld KAM, Kang N, Bienenstock J, Foster JA. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol. 2011;4(4):492-494. https://doi.org/10.4161/cib.4.4.15702
22. Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047-3052. https://doi.org/10.1073/pnas.1010529108
23. McKean J, Naug H, Nikbakht E, Amiet B, Colson N. Probiotics and subclinical psychological symptoms in healthy participants: a systematic review and meta-analysis. J Altern Complement Med. 2017;23(4):249-258. https://doi.org/10.1089/acm.2016.0023
24. Rhee SH, Keates AC, Moyer MP, Pothoulakis C. MEK is a key modulator for TLR5-induced interleukin-8 and MiP3α gene expression in non-transformed human colonic epithelial cells. J Biol Chem. 2004;279(24):25179-25188. https://doi.org/10.1074/jbc.m400967200
25. Charrier L, Driss A, Yan Y, et al. hPepT1 mediates bacterial tripeptide fMLP uptake in human monocytes. Lab Invest. 2006;86(5):490-503. https://doi.org/10.1038/labinvest.3700413
26. Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132(5):1890-1901. https://doi.org/10.1053/j.gastro.2007.02.036
27. Agrawal A, Houghton LA, Morris J, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29(1):104-114. https://doi.org/10.1111/j.1365-2036.2008.03853.x
28. O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541-551. https://doi.org/10.1053/j.gastro.2004.11.050
29. Rao AV, Bested AC, Beaulne TM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1(1):6. https://doi.org/10.1186/1757-4749-1-6
30. Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394-1401. https://doi.org/10.1053/j.gastro.2013.02.043
31. Singh RK, Chang HW, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. https://doi.org/10.1186/s12967-017-1175-y
32. Mutlu EA, Gillevet PM, Rangwala H, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G966-G978. https://doi.org/10.1152/ajpgi.00380.2011
33. Berkowitz L, Schultz BM, Salazar GA, et al. Impact of cigarette smoking on the gastrointestinal tract inflammation: opposing effects in Crohn's disease and ulcerative colitis. Front Immunol. 2018;9:74. https://doi.org/10.3389/fimmu.2018.00074
34. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8(3):e59260. https://doi.org/10.1371/journal.pone.0059260
35. Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2016;63:217-227. https://doi.org/10.1016/j.psyneuen.2015.10.001
36. Galley JD, Nelson MC, Yu Z, et al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. https://doi.org/10.1186/1471-2180-14-189
37. Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy. Gastroenterology. 2003;124(5):1202-1209. https://doi.org/10.1016/s0016-5085(03)00171-9
38. Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complement Altern Med. 2010;10:13. https://doi.org/10.1186/1472-6882-10-13
39. Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;2010(11):CD003048. https://doi.org/10.1002/14651858.cd003048.pub3
40. Ruszczyński M, Radzikowski A, Szajewska H. Clinical trial: effectiveness of Lactobacillus rhamnosus (strains E/N, Oxy and Pen) in the prevention of antibiotic-associated diarrhoea in children. Aliment Pharmacol Ther. 2008;28(1):154-161. https://doi.org/10.1111/j.1365-2036.2008.03714.x
41. Dimidi E, Christodoulides S, Fragkos KC, Scott SM, Whelan K. The effect of probiotics on functional constipation: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;100(4):1075-1084. https://doi.org/10.3945/ajcn.114.08915


