Peer Reviewed
Posterior Reversible Encephalopathy Syndrome in a Patient With Hypertension on Oral Methotrexate Therapy
Authors:
Jette Hooper, MD
Transitional Year Resident, Department of Internal Medicine, St. Vincent Hospital and Health Care Center, Indianapolis, Indiana
Anna W. Anderson, MD
Transitional Year Resident, Department of Internal Medicine, St. Vincent Hospital and Health Care Center, Indianapolis, Indiana
Azmina Alibhai, MD
Internal Medicine Resident, Department of Internal Medicine, St. Vincent Hospital and Health Care Center, Indianapolis, Indiana
Michelle Solik, MS, MD
Academic Clinician and Chair of Resident Recruitment, Department of Internal Medicine, St. Vincent Hospital and Health Care Center, Indianapolis, Indiana
Citation:
Hooper J, Anderson AW, Alibhai A, Solik M. Posterior reversible encephalopathy syndrome in a patient with hypertension on oral methotrexate therapy. Consultant. 2019;59(7):199-201.
An 86-year-old woman presented to our institution from an outside hospital for management of altered mental status. She had a history of B-cell lymphoma, for which she had received radiation therapy; rheumatoid arthritis (RA), for which she was on methotrexate; a history of multiple unprovoked venous thromboemboli, for which she was on warfarin; and hypertension.
History. At initial presentation, the patient was unable to provide any meaningful history, given her encephalopathic state; therefore, most of her history was obtained from family. The patient reportedly had been functioning independently in her living facility—completing her activities of daily living with minimal assistance and being oriented and appropriate in her mentation.
However, in the 2 weeks prior to presentation at the outside hospital, the patient had experienced 3 falls. Each fall had prompted an assessment at the local emergency department, with the first 2 falls resulting in a same-day discharge. The most recent fall with associated confusion had led to admission at the outside hospital for further evaluation. At that time, she was found to have had a possible urinary tract infection. There was no evidence of pneumonia or acute intracranial pathology on chest or head imaging, respectively. A computed tomography (CT) scan of the abdomen and pelvis (completed for unclear reasons) revealed a large 9-cm lesion in the spleen, which was a new finding when compared with a prior CT scan. Empiric treatment with ceftriaxone, metronidazole, and vancomycin was initiated due to concern for a possible intra-abdominal infection. However, she had failed to improve and was transferred to our facility for further evaluation.
The patient had no history of recent travel or known toxic exposures. The family denied any use of tobacco, alcohol, or illicit drugs by the woman. Her family history was otherwise noncontributory. Her medical history included B-cell lymphoma, RA, deep vein thrombosis, dementia, hypertension, hyperlipidemia, gout, and gastroesophageal reflux disease. Her medications were methotrexate, acetaminophen, allopurinol, calcium, warfarin, duloxetine, folic acid, gabapentin, lisinopril, hydrocodone, simvastatin, albuterol sulfate, and vitamin B6. She had been on methotrexate for nearly 6 months for management of what the family described as “questionable RA.” She had received a diagnosis of B-cell lymphoma 18 months ago with localization to the right distal femur, which was treated with radiation therapy, most recently 17 months ago. A magnetic resonance imaging (MRI) scan performed 3 weeks prior to admission had shown improvement in this regard.
Physical examination. At the current presentation, the patient was afebrile. Her vital signs were within normal limits other than an elevated blood pressure of 183/108 mm Hg on arrival, which was fairly consistent with charted blood pressures at the outside hospital. She had a positive pharyngeal reflex. No obvious abnormalities were noted on examination of the head, eyes, ears, nose, and throat. Cardiovascular examination did not reveal any murmurs. Her lungs were clear to auscultation bilaterally. Abdominal examination revealed morbid obesity, but the abdomen was otherwise soft, without evidence of guarding or rebound, and with no obvious hepatosplenomegaly. Skin examination findings were unremarkable. There was no evidence of synovitis on joint examination, and the lower extremities were nonedematous. There was no appreciable lymphadenopathy. Neurological examination was challenging given her mental state, but she moved all extremities spontaneously and would open her eyes to voice and sternal rub.
Diagnostic tests. Pertinent laboratory data on admission were as follows: elevated white blood cell (WBC) count of 19,200/µL (reference range, 3300-10,500/µL); corrected calcium, 11.7 mg/dL (reference range, 8.4-10.2 mg/dL); blood urea nitrogen, 31 mg/dL (reference range, 9-20 mg/dL); creatinine, 1.23 mg/dL (reference range, 0.66-1.25 mg/dL); alanine aminotransferase, 54 U/L (reference range, 11-58 U/L); aspartate aminotransferase, 107 U/L (reference range, 17-59 U/L); C-reactive protein, 6.5 mg/L (reference range, 0.1-0.8 mg/L); lactate, 1.1 mmol/L (0.5-2.2 mmol/L); and procalcitonin, 0.26 ng/mL (reference value, <0.10 ng/mL). Urinalysis results were negative for nitrite and leukocyte esterase, with mild proteinuria (non-nephrotic range). A chest radiograph did not reveal any acute pathology. Blood cultures collected at the outside hospital remained negative for pathogens throughout the patient’s hospital course.
Diagnosis. The initial differential diagnosis included toxic encephalopathy secondary to infection (urinary tract infection vs intra-abdominal process vs meningitis vs encephalitis), metabolic encephalopathy secondary to hypercalcemia, or progression of B-cell lymphoma. The patient had been treated with broad-spectrum antibiotics for several days prior to her arrival at our hospital and had made little improvement, so infection seemed unlikely. Repeated chest and abdominal imaging and urinalysis at our institution did not reveal any evidence of recurrent or worsening infection. The patient’s serum calcium and creatinine levels were elevated, so aggressive hydration with intravenous fluids was initiated. Her calcium and creatinine levels subsequently normalized within the first 24 hours of admission, yet her encephalopathy remained unchanged. It should be noted that her medications, including methotrexate, were held during evaluation.
A lumbar puncture (LP) was performed following repeated head CT in an effort to elucidate whether encephalitis or meningitis were contributing to her presentation. LP revealed a protein level of 127 mg/dL (reference range, 12-60 mg/dL), a WBC count of 6/µL (reference range, 0-5/µL), and a glucose level of 67 mg/dL (reference range, 40-70 mg/dL). Cerebrospinal fluid (CSF) analysis was negative for Lyme disease, syphilis, West Nile virus, Cryptococcus, enterovirus, histoplasmosis, acid-fast organisms, and herpes simplex viruses 1 and 2. CSF bacterial and viral cultures showed no growth. Cytology results were negative for malignant cells but showed rare monocytes and small lymphocytes (making progression of B-cell lymphoma less likely). Although an argument could have been made for possible viral meningitis in light of the mildly elevated CSF protein level (although, again, analysis for viral etiologies returned largely negative), this seemed unlikely.
On hospital day 3, MRI with and without contrast revealed an extensive signal abnormality within the white matter of the posterior cerebral hemispheres and frontal lobes, cerebellar hemispheres, and thalami (Figure), a finding most consistent with posterior reversible encephalopathy syndrome (PRES).
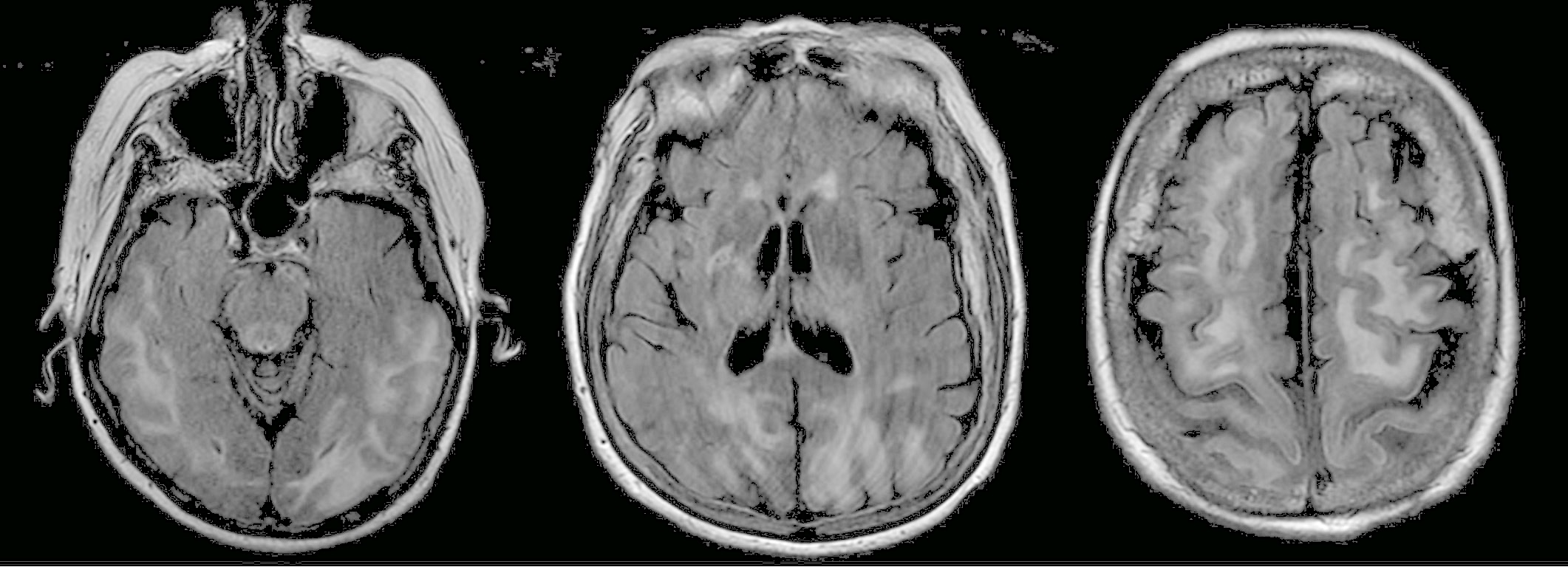
Figure. Extensive hyperintense signal was seen on T2-weighted fluid-attenuated inversion recovery MRI images involving the subcortical and juxtacortical white matter of the posterior temporal, occipital, parietal, and frontal lobes in addition to the thalami.
Treatment and outcome. In light of the MRI findings suggestive of PRES, aggressive blood pressure control measures were pursued with intravenous hydralazine and enalapril, which resulted in an observed dramatic improvement in the patient’s level of encephalopathy. Within days, she was back to her baseline mentation and had successfully transitioned to oral antihypertensive medications with the achievement of tight blood pressure control. She was nearing discharge but had an unforeseen outcome related to administration of therapeutic enoxaparin for management of her history of chronic venous thromboembolism, which led to hemorrhagic shock and her ultimate death.
Discussion. PRES, also known as posterior reversible leukoencephalopathy syndrome, is a rare entity that primarily causes reversible vasogenic edema without infarction in the posterior circulation of the brain but can also include the anterior circulation and infratentorial regions.1,2 PRES was first described in 1996 by Hinchey as a variable clinical syndrome associated with characteristic neuroimaging findings.3 The pathogenesis is thought to be high blood pressure overcoming cerebral autoregulation leading to capillary leakage.4
The medical literature on PRES is largely limited to case series and case reports. The spectrum of presenting symptoms can include altered mental status, seizures, headache, visual disturbances, and focal neurological deficits.5 The authors of a recent case series of 13 patients found that the most common clinical manifestation of PRES was seizures, which were observed in 10 of the 13 patients.6 The authors also noted that the most common comorbidity (seen in 11 of the patients with PRES) was chronically impaired renal function.6 Neither of these factors were present in our patient. The more common risk factors for PRES are hypertension and eclampsia but also include the use of immunosuppressive agents (such as cyclosporine, tacrolimus, interferon-alfa, and intrathecal methotrexate), infections, autoimmune disease, and trauma.5,7,8 Methotrexate has been described in the literature as a culprit in cases of PRES but only when used intrathecally.9,10 Our patient had been on oral methotrexate in the months leading up to presentation, and her family had been unaware of her use of any other alternative immune-system modulators in the management of her RA.
Imaging plays an important role in the diagnosis of PRES. MRI is more sensitive in diagnosing PRES than CT, and the authors of one case series of 30 patients found that 37% presented with negative findings on head CT.11 Therefore, MRI is the preferred imaging modality for diagnosis of suspected PRES.12 Imaging should be implemented early in the evaluation of patients on immunosuppressive agents who present with altered mental status and hypertension. Typical MRI findings consistent with PRES are observed primarily in the parieto-occipital region of the brain; however, atypical presentations often include the frontal lobe, basal ganglia, cerebellum, or temporal lobes.13 Radiologists who note such changes on an MRI should immediately discuss the possibility of PRES with their clinician colleagues so that the patient’s blood pressure may be adequately controlled.
PRES is nearly 85% reversible, with strict blood pressure control and the discontinuation of immunosuppressive agents being the key components of management.8,11 Nevertheless, our patient died as a result of alternative complications despite successful blood pressure control and initial improvement in her symptoms.
References:
- Liman T, Bohner G, Heuschmann P, Endres M, Siebert E. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: the retrospective Berlin PRES study. J Neurol. 2011;259(1):155-164.
- Chen Z, Zhang G, Lerner A, Wang A-H, Gao B, Liu J. Risk factors for poor outcome in posterior reversible encephalopathy syndrome: systematic review and meta-analysis. Quant Imaging Med Surg. 2018;8(4):421-43
- Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
- Stott VL, Hurrell MA, Anderson TJ. Reversible posterior leukoencephalopathy syndrome: a misnomer reviewed. Intern Med J. 2005;35(2):83-90.
- Pereira PR, Pinho J, Rodrigues M, et al. Clinical, imagiological and etiological spectrum of posterior reversible encephalopathy syndrome. Arq Neuropsiquiatr. 2015;73(1):36-40.
- Pirola JP, Baenas DF, Haye Salinas MJ, et al. Posterior reversible leukoencephalopathy syndrome: case series and review of the literature [published online May 31, 2018]. Reumatol Clin. doi:10.1016/j.reuma.2018.04.00
- Lee VH, Wijdicks EFM, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
- Garg RK. Posterior leukoencephalopathy syndrome. Postgrad Med J. 2001;77(903):24-2
- Mescher C, Slungaard A. Posterior reversible encephalopathy syndrome in a postpartum woman with acute lymphoblastic leukaemia after intrathecal methotrexate [published online October 4, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-22042
- Pavlidou E, Pavlou E, Anastasiou A, et al. Posterior reversible encephalopathy syndrome after intrathecal methotrexate infusion: a case report and literature update. Quant Imaging Med Surg. 2016;6(5):605-611.
- Singer S, Grommes C, Reiner AS, Rosenblum MK, DeAngelis LM. Posterior reversible encephalopathy syndrome in patients with cancer. Oncologist. 2015;20(7):806-8
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
- Raman R, Devaramane R, Jagadish GM, Chowdaiah S. Various imaging manifestations of posterior reversible encephalopathy syndrome (PRES) on magnetic resonance imaging (MRI). Pol J Radiol. 2017;82:64-70.


